Update of a Placebo-Controlled Trial of C1 Esterase Inhibitor for Prevention of Antibody Mediated Rejection (ABMR) in Highly-HLA Sensitized Patients.
Kidney Transplant, Cedars-Sinai Medical Center, LA, CA
Meeting: 2017 American Transplant Congress
Abstract number: A50
Keywords: Highly-sensitized, HLA antibodies, IVIG, Kidney transplantation
Session Information
Session Name: Poster Session A: Clinical Science: Kidney Immunosuppression: Desensitization
Session Type: Poster Session
Date: Saturday, April 29, 2017
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall D1
INTRODUCTION: Desensitization (DES) strategies focus primarily on antibody reduction using IVIG, rituximab ± PLEX to prevent DSA-induced ABMR and rebound B-cell activity. Pathologic features of ABMR suggest an important role for complement dependent injury that is often associated with poor outcomes. Thus, complement inhibitors (anti-C5 & C1INH), aimed at reducing or eliminating complement activation by DSAs have shown promise. C1INH is a serpin (serine protease inhibitor) that inhibits the Classic and Mannose Binding Lectin Pathway. Here we report long-term outcomes of a placebo-controlled, single center study using C1INH for prevention of ABMR (NCT:01134510; IND:14363). PATIENTS & METHODS: From 12/2011- 4/2013, 20 HS patients (2LD/8DD in each group) DES with IVIG + rituximab ± PLEX were enrolled and randomized 1:1 to receive C1INH {G1} (20U/kg/dose) vs. Placebo {G2} intra-operatively, then 2x weekly x7 doses. All patients had FCMX+ or DSA+ @transplant and 18/20 underwent protocol biopsy @6M. RESULTS: In G1, 2 patients (20%) developed ABMR. Both patients were treated with pulse steroid+IVIG+rituximab. In G2, 3 patients developed ABMR, that were more severe and had higher DSAs and chronicity than G1. Complications included: in G1, 1 patient developed BKAN @6M and 1 patient with sclerosing encapsulating peritonitis resulted in death secondary to intractable septic shock @36M; in G2, 1 patient died of hyperkalemia during HD catheter insertion @1M and 1 patient developed West Nile Virus infection @36M. Patient survival was 90% in each group. Death censored graft survival was 100% in G1 v. 90% G2. eGFR @36M was 77±22 in G1 vs. 65±21 (ml/min) in G2 (P=NS) {Figure 1}. CONCLUSIONS: Overall patients in both groups continue to do well out to 36M. No patient in G1 developed de novo DSA and only 1 developed TG. In the G2,1 patient developed de novo DSA and anti-AT1R ab and 2 patients developed TG. The death in G1 was deemed unrelated to C1INH therapy. DSA levels were lower in C1INH patients. Larger studies would be helpful to further define the benefits of C1INH in prevention of ABMR.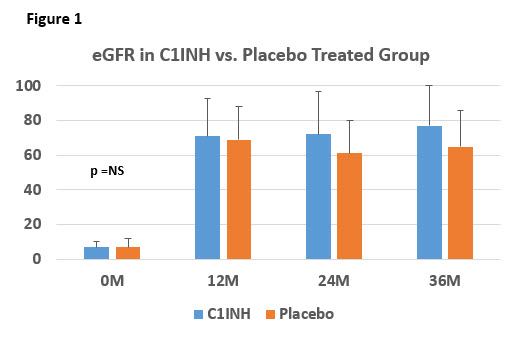
CITATION INFORMATION: Vo A, Choi J, Peng A, Lim K, Varanasi L, Najjar R, Huang E, Puliyanda D, Jordan S. Update of a Placebo-Controlled Trial of C1 Esterase Inhibitor for Prevention of Antibody Mediated Rejection (ABMR) in Highly-HLA Sensitized Patients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Vo A, Choi J, Peng A, Lim K, Varanasi L, Najjar R, Huang E, Puliyanda D, Jordan S. Update of a Placebo-Controlled Trial of C1 Esterase Inhibitor for Prevention of Antibody Mediated Rejection (ABMR) in Highly-HLA Sensitized Patients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/update-of-a-placebo-controlled-trial-of-c1-esterase-inhibitor-for-prevention-of-antibody-mediated-rejection-abmr-in-highly-hla-sensitized-patients/. Accessed February 27, 2026.« Back to 2017 American Transplant Congress
