Tocilizumab in Chronic Active Antibody-Mediated Rejection in Kidney Transplant Recipients: A Case Series
1Nephrology, University of Washington, Seattle, WA, 2Surgery, University of Washington, Seattle, WA, 3Bloodworks Northwest, Seattle, WA
Meeting: 2022 American Transplant Congress
Abstract number: 1407
Keywords: Graft survival, Kidney transplantation, Rejection, Renal function
Topic: Clinical Science » Kidney » 45 - Kidney Chronic Antibody Mediated Rejection
Session Information
Session Name: Kidney Chronic Antibody Mediated Rejection
Session Type: Poster Abstract
Date: Monday, June 6, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The optimal treatment for chronic active antibody-mediated rejection (ca-AMR) remains unclear. Tocilizumab (TCZ), a monoclonal antibody against IL-6, has been proposed in ca-AMR treatment to reduce the inflammation and injury without significant side effects. We reported our experience with treatment of ca-AMR with TCZ either as the first line option or as a rescue therapy.
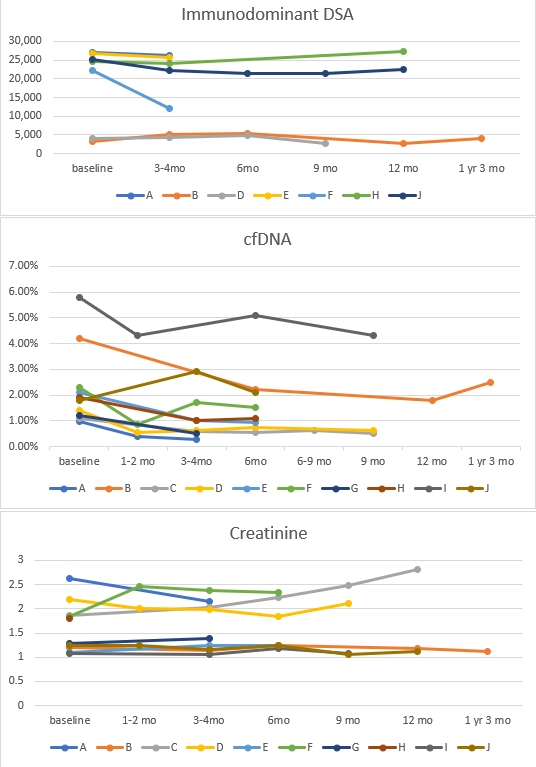
*Methods: 10 adult kidney transplant recipients with biopsy-proven ca-AMR who were treated with TCZ (8 mg/kg IV monthly) were enrolled. Creatinine, urinary protein/creatinine ratio, donor-specific antibody (DSA) and donor-derived cell-free DNA percentage (%dd-cfDNA) and side effects were monitored at least every 3 months.
*Results: In this cohort, mean age of patients was 47 years. CA-AMR was diagnosed at a median of 8.3 years (range 1.2-19 years) post-transplant. Patient received a minimum of 3 months of TCZ. Median duration of treatment was 6.5 months (range 3-12 months). At 3 months, creatinine. DSA and %dd-cfDNA remained stable but proteinuria reduced significantly (average reduction 47% ± 31%, p=0.02). At 6 months, %dd-cfDNA reduced significantly by 66% ± 23% (p=0.01) while Cr and DSA remained unchanged (change Cr 0.01 ± 0.26, change in DSA -267 ± 3100 MFI, p>0.05). All patients had elevated dd-cfDNA≥1% and with treatment in 6/10 the dd-cfDNA decreased below the threshold of <1%. Only one patient experienced a severe infection episode during treatment (C difficile colitis). Overall no patient has lost their grafts in this cohort.
*Conclusions: In our early short-term experience, TCZ appears to reduce graft injury as seen by %dd-cfDNA and proteinuria. There was no worsening of allograft function and DSA remained unchanged. No significant side effect was observed in our cohort.
| Age (mean, SD) | 47 +/- 11 years |
| Living donor transplant | 60% |
| Deceased donor transplant | 30% |
| Simultaneous kidney-pancreas transplant | 10% |
| Time to diagnosis from transplant | 99 months (range 14-227) |
| Previous treatment for AMR/CA-AMR | 30% (IVIG +/- Rituximab) |
| Positive DSA at the time of diagnosis | 70% |
| C4d positive | 70% |
To cite this abstract in AMA style:
Boonpheng B, Bakthavatsalam R, Blosser CD, Castro ICDe, Gimferrer I, Ng Y, Leca N. Tocilizumab in Chronic Active Antibody-Mediated Rejection in Kidney Transplant Recipients: A Case Series [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/tocilizumab-in-chronic-active-antibody-mediated-rejection-in-kidney-transplant-recipients-a-case-series/. Accessed July 18, 2025.« Back to 2022 American Transplant Congress