Thymoglobulin Efficacy: The Role of Dosing on Viral Infection and Rejection in Pancreas Transplantation
Transplant Surgery, University of Minnesota Medical Center, Minneapolis, MN
Meeting: 2019 American Transplant Congress
Abstract number: D269
Keywords: Induction therapy, Infection, Pancreas, Rejection
Session Information
Session Name: Poster Session D: Pancreas and Islet: All Topics
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: While T-cell depletion therapy is standard practice for induction in pancreas transplantation, little data exists to help determine dosing and its impact on efficacy and adverse effects. Our center uses Thymoglobulin® (TMG) for induction with a goal cumulative dose of 6.25 mg/kg for simultaneous pancreas and kidney (SPK) and 8.75 mg/kg for pancreas transplant alone (PTA) and pancreas after kidney (PAK). Doses are reduced or held based on absolute lymphocyte count, WBC and PLT count, but additional doses may be given per clinical discretion. The primary objective was to evaluate the effect of TMG dosing on biopsy proven acute cellular rejection (BPAR) and viral infection (CMV, EBV and BK) within six months.
*Methods: A retrospective review of 179 adult pancreas transplants performed at our center from 4/22/2011 through 8/24/2017 was conducted. Patients were divided into two groups: SPK and PTA. PTA included any solitary pancreas transplant irrespective of previous kidney or pancreas transplant. Total TMG received ranged from 1.27 to 9.62 mg/kg in SPK recipients and 3.98 to 10.22 mg/kg in PTA recipients. Each group was then stratified evenly into three categories. Exclusion criteria included graft pancreatectomy within ten days of transplant (n=6) and history of lung transplant (n=3). For patients with multiple transplants within the time period, the last transplant was utilized. The final data set included 167 patients comprised of 58.6% SPK and 41.3% PTA recipients. Crossmatches were negative, and there was no significant difference in cPRAs between groups. Overall there were 87.4% Caucasian, 50.3% female and 30.5% with a previous transplant; the only significant difference among groups was a higher percentage of females in the SPK high TMG group.
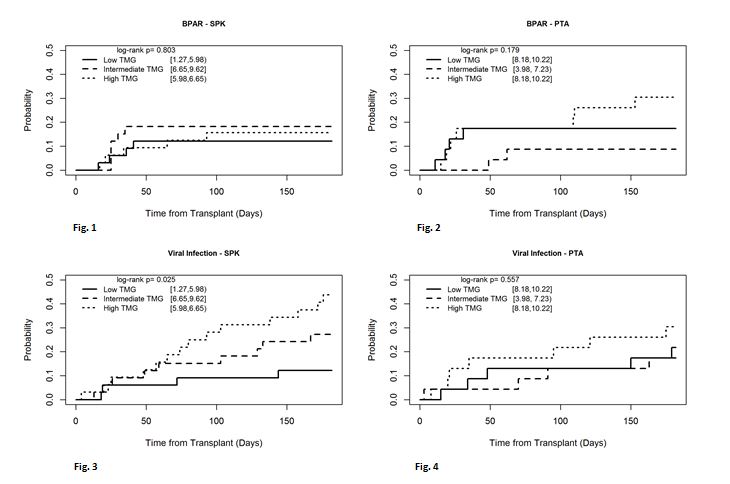
*Results: There were two graft losses in the PTA group within six months: one due to thrombosis (low TMG group), the other due to rejection (intermediate TMG group). In SPK and PTA recipients the incidence of BPAR was not significantly different among the low, intermediate, or high TMG groups (Fig 1 & 2). SPK recipients in the high TMG group had a higher incidence of viral infection (Fig 3, p=0.025). The differences were not significant in the PTA group (Fig 4, p=0.557).
*Conclusions: Higher doses of induction therapy with TMG had an increased risk of viral infection in SPK recipients but did not confer a decreased risk of rejection. Additional studies are warranted as there is no clear optimal dose of TMG and dose reduction could result in fewer viral complications as well as cost benefit.
To cite this abstract in AMA style:
Sarumi H, Johnson B, Jackson S, Hogan S, Fisher J, Riad S, Kandaswamy R, Pruett T. Thymoglobulin Efficacy: The Role of Dosing on Viral Infection and Rejection in Pancreas Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/thymoglobulin-efficacy-the-role-of-dosing-on-viral-infection-and-rejection-in-pancreas-transplantation/. Accessed March 3, 2026.« Back to 2019 American Transplant Congress