Three-Way Comparison of Central DSA, %dd-cf DNA, and Molecular Biopsy Findings in the Trifecta Study
1Alberta Transplant Applied Genomics Centre, Edmonton AB, AB, Canada, 2University of Wisconsin, Madison, WI, 3Natera Inc., San Carlos, CA, 4One Lambda Inc., West Hills, CA, 5., ., AB, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 121
Keywords: Biopsy, HLA antibodies, Kidney transplantation
Topic: Clinical Science » Kidney » 45 - Kidney Chronic Antibody Mediated Rejection
Session Information
Session Name: Kidney Complications: Chronic Antibody Mediated Rejection & Immune Mediated Late Graft Failure
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:50pm-7:00pm
Presentation Time: 6:50pm-7:00pm
Location: Hynes Room 302
*Purpose: Trifecta (ClinicalTrials.gov #NCT04239703) is a prospective, IRB-approved, consented study of the relationships among three central tests in kidney transplant indication biopsies: molecular biopsy diagnoses (Molecular Microscope® Diagnostic System; MMDx), %dd-cfDNA, and HLA antibody testing. This report analyzes all three results.
*Methods: MMDx was assessed at ATAGC; %dd-cfDNA by Natera Inc. (Prospera); central HLA antibody testing by One Lambda Inc. LGH interpreted HLA antibody as DSA using local donor-recipient genotyping. Local standard-of-care histology and DSA were recorded. Some DSA-negative HLA-antibody (âPRAâ) positive biopsies were flagged as high risk (âDSANHRâ) due to incomplete donor class II genotyping.
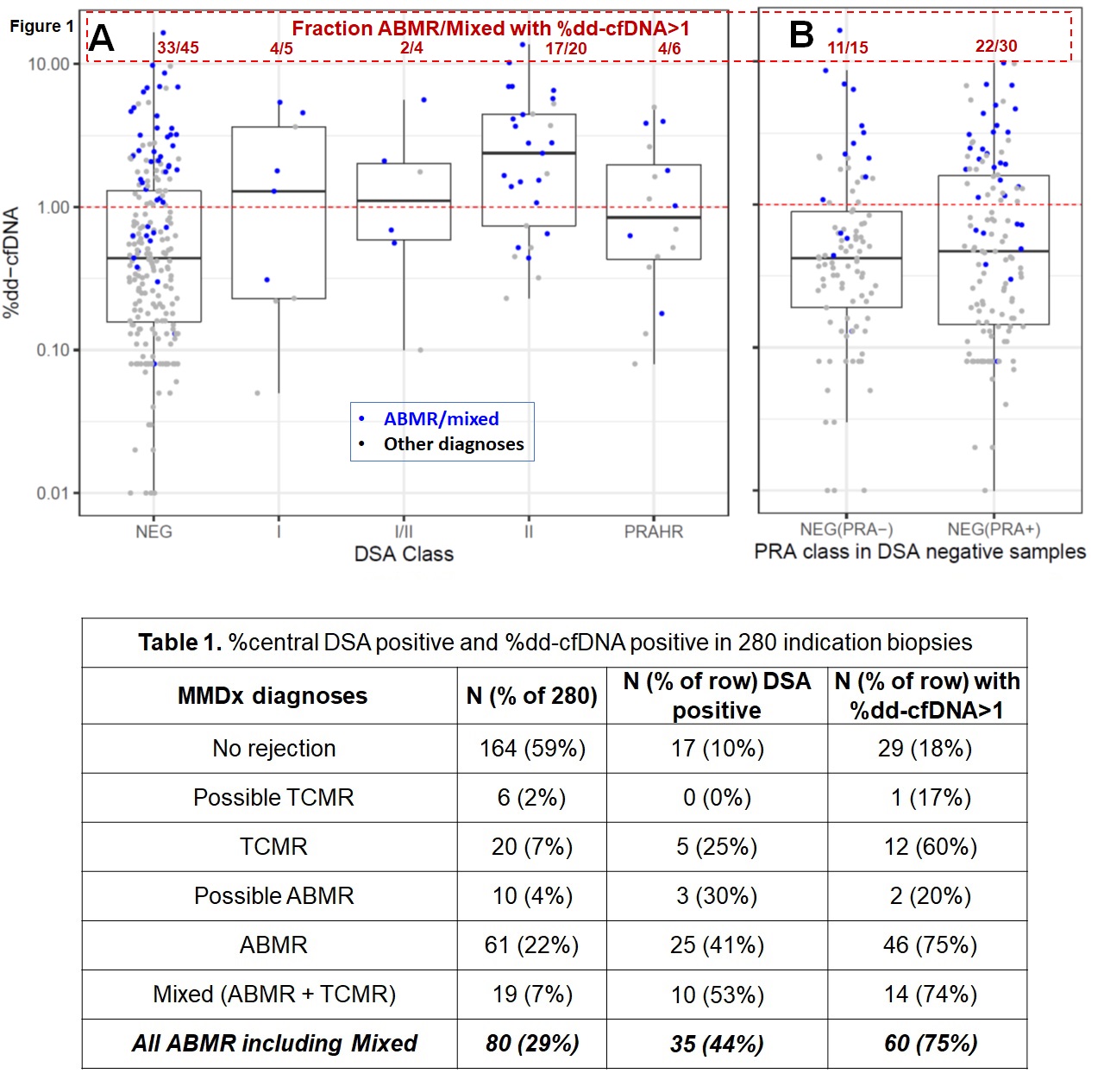
*Results: Central DSA was associated with elevated %dd-cfDNA: geometric mean 0.45% in DSA-negative vs. 1.28% in DSA-positive (p=3.7×10-7, using log %dd-cfDNA). Most samples called DSA-negative locally were confirmed DSA-negative centrally (113/118 (96%)). By MMDx diagnoses (Table 1; Figure 1A), 35/80 ABMR/Mixed biopsies (44%) were DSA-positive (including 6 DSANHR). Histology was similar: 29/59 ABMR/mixed DSA-positive (49%), Most DSA-negative ABMR/Mixed were sensitized: 30/45 (67%) were PRA+ve. In ABMR/mixed, 60/80 (75%) had %dd-cfDNA>1.0: 33/45 (73%) DSA-negative and 27/35 (77%) DSA-positive. Thus, high %dd-cfDNA was as frequent in DSA-negative as in DSA-positive ABMR/mixed (p=0.90). In Figure 1B, 11/22 DSA-negative ABMR with high %dd-cfDNA (67%) were PRA-positive. In logistic regression, continuous %dd-cfDNA measurements predicted ABMR/mixed better than DSA status (AIC 292.42 vs. 332.93 – smaller AICs are better). Log(%dd-cfDNA) added significantly to a model with DSA alone (p=4×10-12). DSA added significantly to cfDNA alone (p=0.008). The best predictive model for biopsy ABMR/mixed used both %dd-cfDNA and DSA together.
*Conclusions: In the first 280 triple results (central DSA, %dd-cfDNA, and biopsy MMDx), 75% of molecular ABMR/mixed biopsies had %dd-cfDNA>1.0. Only 44% of ABMR/mixed were DSA-positive. However, most DSA-negative ABMR/mixed were sensitized (67% PRA+ve). Local and central DSA-negative results were similar. Thus %dd-cfDNA is a robust feature of active ABMR, regardless of DSA status. In regression, the best prediction of ABMR activity at the time of indication biopsy includes both %dd-cfDNA and DSA.
To cite this abstract in AMA style:
Halloran PF, Hidalgo LG, Demko Z, Prewett A, Reeve J, Lawrence C, Lowe D, Billings P. Three-Way Comparison of Central DSA, %dd-cf DNA, and Molecular Biopsy Findings in the Trifecta Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/three-way-comparison-of-central-dsa-%dd-cf-dna-and-molecular-biopsy-findings-in-the-trifecta-study/. Accessed December 28, 2025.« Back to 2022 American Transplant Congress