This is Why We Can't Have Nice Things- Real World Experience with Everolimus in Livers.
1Pharmacy, Medical University of South Carolina, Chareleston, SC
2Surgery, Medical University of South Carolina, Charleston, SC
Meeting: 2017 American Transplant Congress
Abstract number: D207
Keywords: Adverse effects, Immunosuppression, Liver
Session Information
Session Name: Poster Session D: Liver: Immunosuppression and Rejection
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Purpose: To determine if discontinuation rates of everolimus (RAD) in clinical practice is higher than the 30% demonstrated in trials.
Methods: This was a retrospective cohort study of adult OLTx patients between Jan 2010 through Dec 2015 who were started on RAD following CNI minimization or withdrawal protocol. Multiorgan transplant patients were excluded. The primary outcome was discontinuation rate of RAD. Other outcomes were days to conversion, reason for initiation, SCr at initiation and last follow-up, rejection, reason for discontinuation, number of days on RAD, and lipid, proteinuria, and CBC monitoring.
Results: A total of 42 patients were included. The median time to conversion was 56 days with the most common indication being malignancy. CNI minimization was the most common protocol used. 21 patients had at least one episode of rejection (Table 2).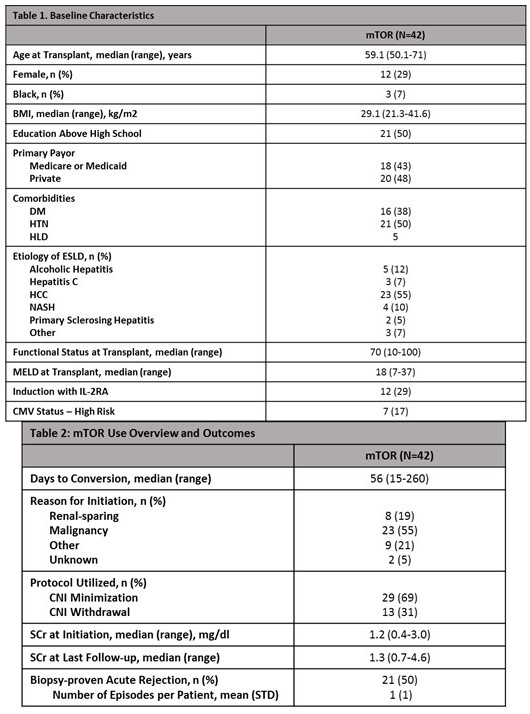 A large proportion of patients discontinued therapy (60%). The most common reasons for discontinuation were rejection and planned surgical procedure. Patients discontinued off RAD were on therapy for a median of 112 days (Table 3). Only 64% of patients got a lipid panel and 41% got a urine protein/Cr ratio checked at initiation. Follow-up lipid panels and urine protein/Cr ratios were only performed in 38% and 12% of patients, respectively. Up to 43% of patients experienced bone marrow suppression (Table 4).
A large proportion of patients discontinued therapy (60%). The most common reasons for discontinuation were rejection and planned surgical procedure. Patients discontinued off RAD were on therapy for a median of 112 days (Table 3). Only 64% of patients got a lipid panel and 41% got a urine protein/Cr ratio checked at initiation. Follow-up lipid panels and urine protein/Cr ratios were only performed in 38% and 12% of patients, respectively. Up to 43% of patients experienced bone marrow suppression (Table 4).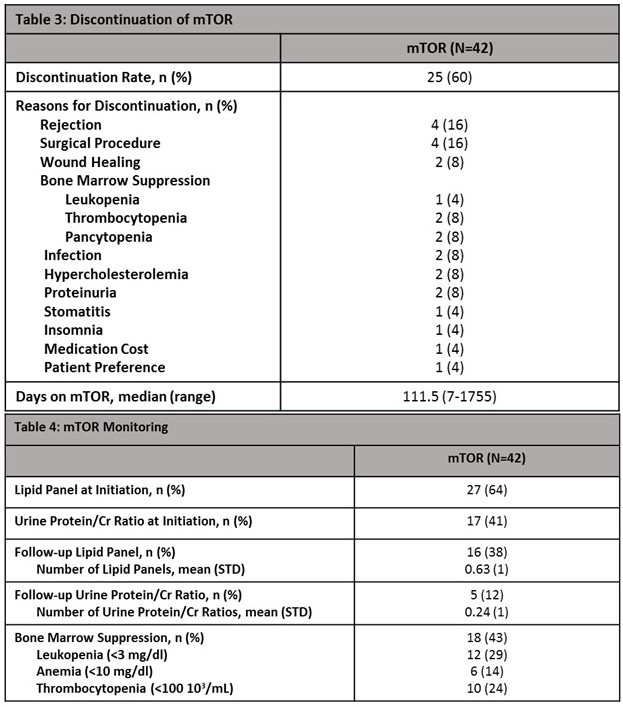 Conclusion: A factor that could be playing a role into the discontinuation of RAD is patients are being discontinued for reasons not related to tolerability and not being placed back on once their issue has resolved. Patients aren't being properly monitored for AEs and many are likely being continued on therapy inappropriately.
Conclusion: A factor that could be playing a role into the discontinuation of RAD is patients are being discontinued for reasons not related to tolerability and not being placed back on once their issue has resolved. Patients aren't being properly monitored for AEs and many are likely being continued on therapy inappropriately.
CITATION INFORMATION: Perez C, Fleming J, Sell M, O'Brien B, Rogers A, Lee I, Mardis C, Mardis A, Patel N, Meadows H, Pilch N, Chavin K, Dubay D, Taber D. This is Why We Can't Have Nice Things- Real World Experience with Everolimus in Livers. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Perez C, Fleming J, Sell M, O'Brien B, Rogers A, Lee I, Mardis C, Mardis A, Patel N, Meadows H, Pilch N, Chavin K, Dubay D, Taber D. This is Why We Can't Have Nice Things- Real World Experience with Everolimus in Livers. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/this-is-why-we-cant-have-nice-things-real-world-experience-with-everolimus-in-livers/. Accessed March 6, 2026.« Back to 2017 American Transplant Congress
