Third Dose of SARS-CoV-2 mRNA Vaccines Significantly Increases Delta (B.1.617.2) Variant Anti-Spike Antibody Levels in Kidney Transplant Recipients
A. Al Jurdi1, R. B. Gassen1, T. Borges1, I. T. Lape1, L. Morena1, O. Efe1, Z. Solhjou2, R. El Fekih2, C. Kotton1, J. Azzi2, L. Riella1
1Massachusetts General Hospital, Boston, MA, 2Brigham and Women's Hospital, Boston, MA
Meeting: 2022 American Transplant Congress
Abstract number: 1634
Keywords: COVID-19, Kidney transplantation, Multicenter studies, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) IV
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Emerging SARS-CoV-2 variants may be associated with a higher risk of breakthrough infections compared to wild-type (WT) virus in kidney transplant recipients (KTRs). The purpose of this study was to evaluate antiviral immune responses against WT and Delta (B.1.617.2) variant of SARS-CoV-2 after 3 doses of SARS-CoV-2 mRNA vaccines in KTRs.
*Methods: We conducted a multicenter prospective cohort study of adult KTRs who received 3 doses of BNT162b2 or mRNA-1273. Blood samples were collected from KTRs before and 4 weeks after the 3rd vaccine dose. Sera from pre-pandemic healthy controls (HCs) and pre-pandemic kidney transplant control patients (KCs) were used for comparison. A Luminex-based multiplex assay was used to measure anti-spike antibodies for the WT, Alpha, Beta, Gamma and Delta variants of SARS-CoV-2. A surrogate virus neutralization test was used to assess neutralization against the WT and Delta variant. Patients were also monitored for rejection using several non-invasive biomarkers.
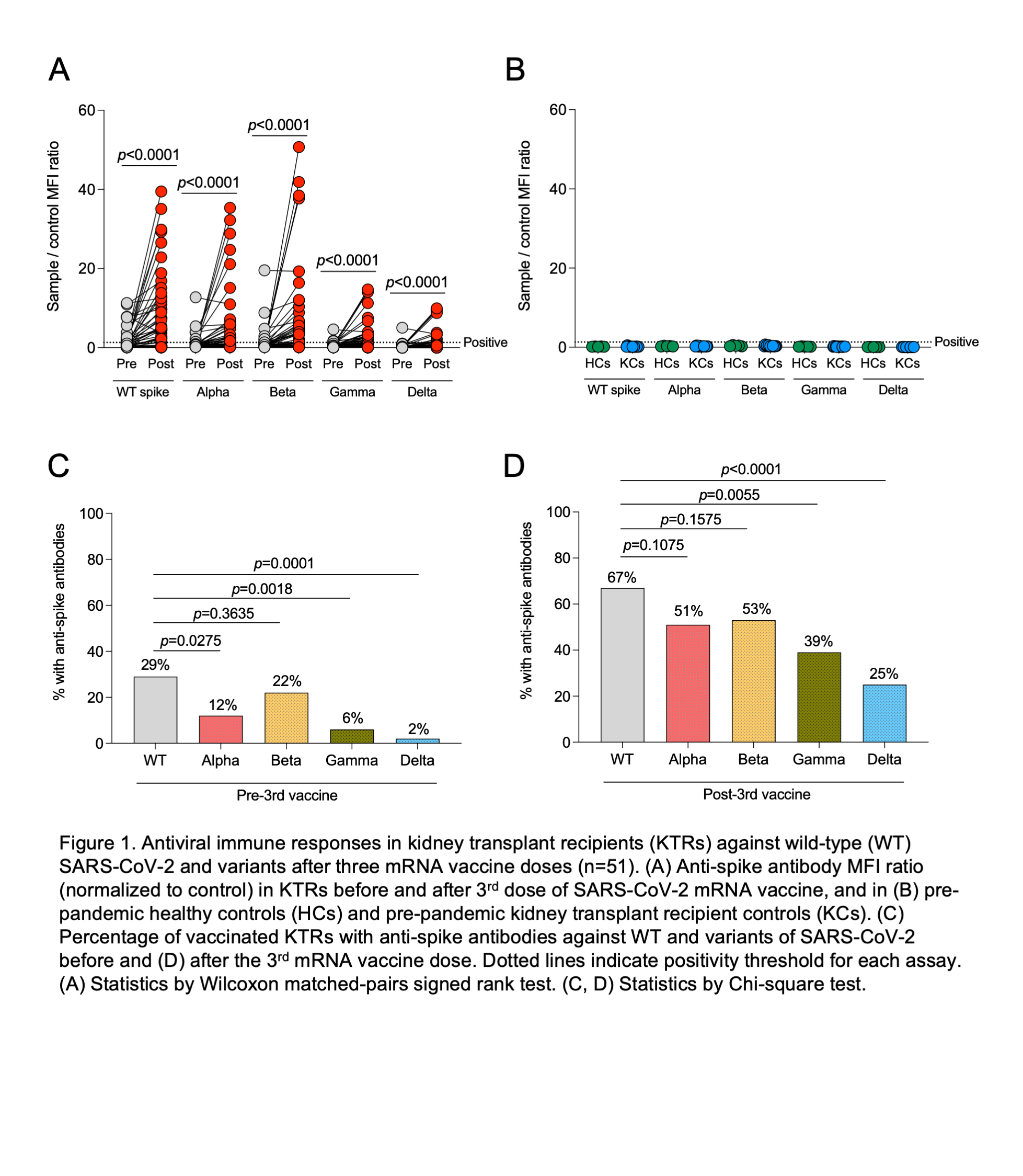
*Results: 54 KTRs were enrolled in the study. The median age was 63, 44% were female and the median time post-transplantation was 42 months. 94% received BNT162b2 vaccine. After the 3rd vaccine dose, there was a significant increase in anti-spike antibody MFIs against the WT, Alpha, Beta, Gamma and Delta variants (Fig. 1A, p<0.0001 for all). For comparison, all pre-pandemic HCs and KCs had a negative result for anti-spike antibody levels (Fig. 1B). Prior to the 3rd vaccine dose, 29% of KTRs had anti-spike antibodies against the WT compared to only 2% against the Delta variant (Fig. 1C, p=0.0001). After the 3rd vaccine dose, 67% of KTRs had anti-spike antibodies against the WT compared to 25% against the Delta variant (p<0.0001, Fig. 1D). Differences between WT and other variants are shown in Figure 1C-D. After the 3rd vaccine dose, there was a 2.1-fold and 2.5-fold increase in the percentage of KTRs with neutralizing responses against the WT and Delta variant respectively (p<0.0001 for both). There was no significant change in serum creatinine, proteinuria, or donor-derived cell-free DNA levels after vaccination. No episodes of rejection occurred during follow-up.
*Conclusions: Two doses of SARS-CoV-2 mRNA vaccines in KTRs are associated with minimal anti-spike antibody response directed against the Delta variant of SARS-CoV-2. After the third dose, a quarter of KTRs developed anti-spike antibodies directed against the Delta variant of SARS-CoV-2.
To cite this abstract in AMA style:
Jurdi AAl, Gassen RB, Borges T, Lape IT, Morena L, Efe O, Solhjou Z, Fekih REl, Kotton C, Azzi J, Riella L. Third Dose of SARS-CoV-2 mRNA Vaccines Significantly Increases Delta (B.1.617.2) Variant Anti-Spike Antibody Levels in Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/third-dose-of-sars-cov-2-mrna-vaccines-significantly-increases-delta-b-1-617-2-variant-anti-spike-antibody-levels-in-kidney-transplant-recipients/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress