The UK ONE Study: Safety and Feasibility of Regulatory T Cell Therapy in Renal Transplantation.
1Transplantation Research Immunology Group, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, United Kingdom
2Oxford Transplant Centre, Oxford, United Kingdom
3Guy's Hospital, London, United Kingdom
4Immunoregulation Laboratory, King's College London, Guy's Hospital, London, United Kingdom
5Department of Surgery, University Hospital Regensburg, Regensburg, Germany.
Meeting: 2016 American Transplant Congress
Abstract number: D311
Keywords: Immunosuppression, Kidney transplantation, T cells, Tolerance
Session Information
Session Name: Poster Session D: Late Breaking
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Introduction: The UK ONE Study trial investigates the safety and feasibility of regulatory T (Treg) cell therapy in renal transplantation.
Materials and Methods: Eleven (9m;2f) living donor renal transplant recipients (RTR), median age 42 (28-71) years, were enrolled and received Treg cell infusions. 350 ml whole blood or 55 ml of leukapheresis product was obtained 6-10 weeks before transplant. Treg were enriched by CD25 positive selection combined with CD8 negative selection and then expanded by stimulation with CD3/CD28 beads + IL-2 in the presence of Rapamycin at the GMP facility. Final cell products were cryopreserved at the specified dose and delivered in a dry shipper (-190[ordm]C). 5 days after transplantation; 2 ml cell product was thawed, suspended in 5% HSA, and administered over 30 minutes as an IV infusion. A dose-escalation of three participants at each of 1×106, 3×106, 6×106 and 10×106 cells/kg was performed. Immunosuppression comprised Mycophenolate Mofetil, Tacrolimus and Prednisolone. No induction therapy was given (Figure 1).
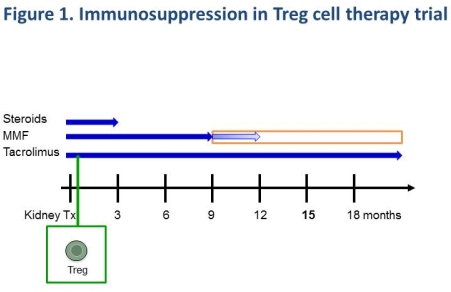
Results and Discussion: No cell-infusion-related adverse events were noted. During a median follow-up of 10 (1-15) months, no biopsy-proven rejection episodes were observed. All patients have stable transplant function (median latest serum creatinine of 128 (90-176) [micro]mol/l).
Conclusion: This study reports the outcome of the first eleven RTR to receive expanded Treg in the UK. The data confirm the feasibility and safety of Treg infusion in RTR to a dose of 10×106 cells per kg. These results demonstrate the feasibility of Treg therapy in solid organ transplantation, and establish the need for future trials investigating the efficacy of Treg therapy in renal transplantation.
CITATION INFORMATION: Bushell A, van der Net J, Game D, Hilton R, Thirkell S, Friend P, Geissler E, Wood K, Harden P, Lombardi G. The UK ONE Study: Safety and Feasibility of Regulatory T Cell Therapy in Renal Transplantation. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Bushell A, Net Jvander, Game D, Hilton R, Thirkell S, Friend P, Geissler E, Wood K, Harden P, Lombardi G. The UK ONE Study: Safety and Feasibility of Regulatory T Cell Therapy in Renal Transplantation. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/the-uk-one-study-safety-and-feasibility-of-regulatory-t-cell-therapy-in-renal-transplantation/. Accessed February 25, 2026.« Back to 2016 American Transplant Congress
