The BEST Trial: A Prospective Randomized Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression
1U Cincinnati, Cincinnati
2U Wisconsin, Madison
3Tampa General, Tampa
4U Colorado, Denver
5U Minnesota, Minneapolis
6UIC, Chicago.
Meeting: 2018 American Transplant Congress
Abstract number: 118
Keywords: Adverse effects, Co-stimulation, Induction therapy, Rejection
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Co-Stimulation Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Room 6C
Previous large multicenter belatacept (BELA)-based CNI- and steroid-free immunosuppressive trials have not enrolled to completion due to safety concerns. The BEST Trial (Belatacept-based Early Steroid Withdrawal Trial) was designed to meet limitations of the pivotal BENEFIT trials by using a tacrolimus (TAC)-based comparator, T cell-depletion, and early steroid withdrawal (ESW) by comparing 2 BELA-based ESW regimens with a TAC-based ESW regimen. 1 year results are presented.
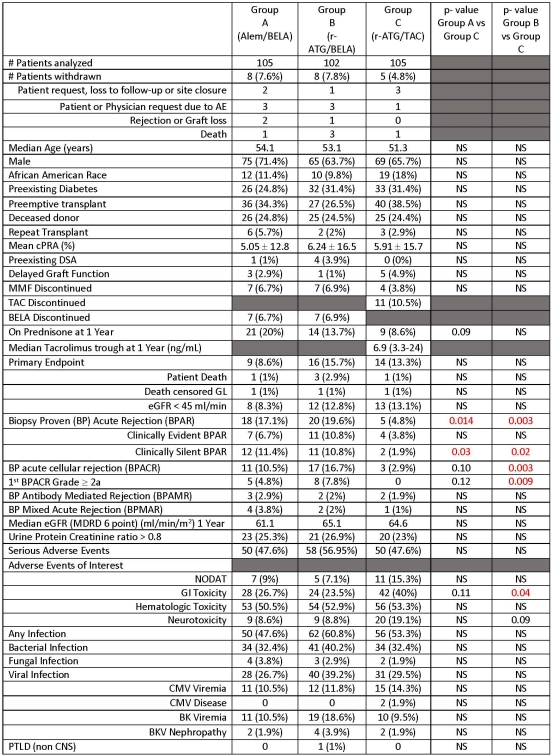
METHODS: This 2 year study was conducted under an FDA IND and IRB at 8 sites. Adult kidney transplant (KTx) patients (pts) were eligible with these exceptions: age < 18 yrs, non-KTx pts, HLA identical living donors, cPRA>50%, ECD KTx pts, Hepatitis B or C or HIV seropositivity, or EBV seronegative pts. All pts received mycophenolate therapy and 5 days of steroids. 315 pts randomized to 3 groups: alemtuzumab + BELA (Group A), r-ATG + BELA (Group B), and r-ATG + TAC (Group C). Primary composite endpoint: patient death or graft loss or eGFR < 45ml/min/m2 at 1 year.
RESULTS: Enrollment was complete on 12/15/16. Intent to treat analyses on 312 pts with 1 year follow-up are presented.
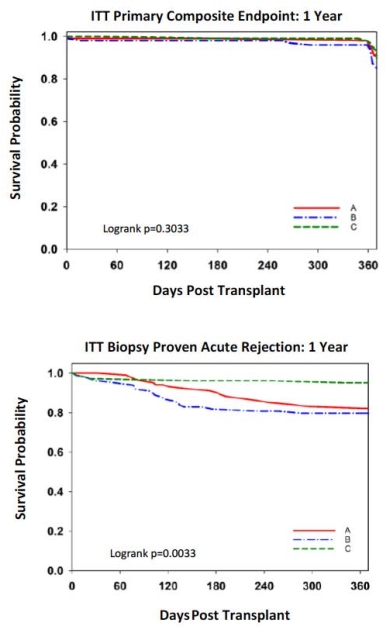
CONCLUSIONS: 1-year results of this 2-year study show that a CNI-free and steroid-free BELA-based protocol is effective and safe 1) Similar primary endpoint rates, 2) Acute rejection rates are higher and of increased histologic severity in BELA pts, but without adverse effects on graft function and survival (data not shown), 3) Similar infections and hematologic toxicity, 4) NODAT, GI toxicity, and neurotoxicity lower in BELA treated pts compared to TAC.
CITATION INFORMATION: Woodle E., Kaufman D., Shields A., Leone J., Wiseman A., Matas A., West-Thielke P., King E., Alloway R. The BEST Trial: A Prospective Randomized Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Woodle E, Kaufman D, Shields A, Leone J, Wiseman A, Matas A, West-Thielke P, King E, Alloway R. The BEST Trial: A Prospective Randomized Multicenter Trial of Belatacept-Based CNI- and Corticosteroid-Free Immunosuppression [abstract]. https://atcmeetingabstracts.com/abstract/the-best-trial-a-prospective-randomized-multicenter-trial-of-belatacept-based-cni-and-corticosteroid-free-immunosuppression/. Accessed December 19, 2025.« Back to 2018 American Transplant Congress