Targeting Calcium Release-Activated Calcium (CRAC) Channel in Non-Human Primate Kidney Transplantation
1Surgery, Duke University, Durham, NC
2Eli Lilly and Company, Indianapolis, IN, Canada
3PRCL Research Inc, Montreal, QC, Canada
4Synta Pharmaceuticals Inc., Lexington, MA.
Meeting: 2018 American Transplant Congress
Abstract number: D37
Keywords: Immunosuppression, Kidney transplantation, T cell activation
Session Information
Session Name: Poster Session D: Immunosuppression Preclinical Studies
Session Type: Poster Session
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
[Background] Long-term administration of calcineurin inhibitors (CNIs) successfully controls rejection of transplanted organs. However, the chronic use of these drugs causes organ damage often resulting in serious kidney damage. Furthermore, chronic rejection of the transplant also occurs despite the use of these drugs. This study was conducted to determine the applicability of a new immunomodulatory drug directed to the calcium release-activated calcium (CRAC) channels of lymphocytes to control transplant rejection using a rhesus monkey renal transplantation model.
[Methods] Animals underwent kidney transplantation and were treated with tacrolimus alone (Target trough: 8-12 ng/ml; n=4), PRCL-02, a CRAC-M1 inhibitor (4-6mg/kg; n=4) alone, or initial tacrolimus with conversion to PRCL-02 (n=3). PRCL-02 was administered via a surgically inserted gastrostomy tube twice daily.
[Results] We determined the dose-related drug exposure in monkeys and then conducted renal transplants using PRCL-02. Oral dosing of PRCL2 (3~30mg/kg/day) was well tolerated. Animals treated with PRCL-02 showed suppressed T cell proliferation comparable to animals with tacrolimus treatment by in vitro MLR. All animals with tacrolimus monotherapy were sacrificed on day 100 without rejection. However, PRCL-02 monotherapy only marginally prolonged graft survival (MST=13.1 days) 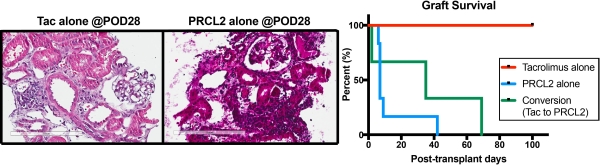 Animals treated with combined PRCL-02 with short-term (21 days) tacrolimus showed MST of 35.3 days, which was prolonged compared to PRCL-02 monotherapy but was not prolonged compared to the tacrolimus-treated group. Pharmacokinetic studies showed inconsistent drug exposures for unknown reasons, which may have contributed to the frequent rejections despite attempts to adjust dose and exposure.
Animals treated with combined PRCL-02 with short-term (21 days) tacrolimus showed MST of 35.3 days, which was prolonged compared to PRCL-02 monotherapy but was not prolonged compared to the tacrolimus-treated group. Pharmacokinetic studies showed inconsistent drug exposures for unknown reasons, which may have contributed to the frequent rejections despite attempts to adjust dose and exposure.
[Conclusion] We conclude that PRCL-02 demonstrated some evidence of immunosuppressive activity but was inferior to tacrolimus with respect to suppressing immune rejection in this transplant model.
CITATION INFORMATION: Kwun J., Ezekian B., Manook M., Park J., Yoon J., Sloan-Lancaster J., Fortier C., Rao P., Knechtle S. Targeting Calcium Release-Activated Calcium (CRAC) Channel in Non-Human Primate Kidney Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Kwun J, Ezekian B, Manook M, Park J, Yoon J, Sloan-Lancaster J, Fortier C, Rao P, Knechtle S. Targeting Calcium Release-Activated Calcium (CRAC) Channel in Non-Human Primate Kidney Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/targeting-calcium-release-activated-calcium-crac-channel-in-non-human-primate-kidney-transplantation/. Accessed February 20, 2026.« Back to 2018 American Transplant Congress
