Tacrolimus Pharmacokinetics (PK) at Low, Medium, and High Trough Levels in Patients Converted from Twice-Daily Immediate-Release Tacrolimus (TacBID) to Once-Daily Extended-Release Tacrolimus (LCPT)
1Service De Néphrologie, Hémodialyse, Aphérèses Et Transplantation Rénale, Chu Grenoble Alpes, La Tronche, France
2Nephrology, University of California, Los Angeles, Los Angeles, CA
3Veloxis, Cary, NC.
Meeting: 2018 American Transplant Congress
Abstract number: 522
Keywords: Calcineurin, Immunosuppression, Kidney, Pharmacokinetics
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: General Considerations - 2
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:30pm-4:42pm
Presentation Time: 4:30pm-4:42pm
Location: Room 6A
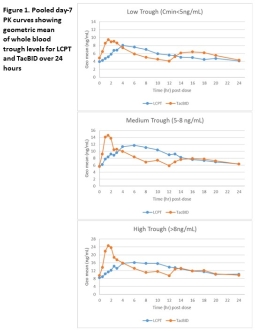
LCPT (Envarsus XR®) is a once-daily, extended-release formulation of tacrolimus which has demonstrated greater bioavailability and a smoother PK profile compared to TacBID. This post-hoc analysis of previous conversion studies summarizes the PK profile of LCPT and TacBID when targeted at low, medium, and high trough levels in kidney transplant recipients. Methods: Data from 3 PK studies on conversion from TacBID to LCPT (Gaber 2013, Tremblay 2016, Trofe-Clark 2017) were pooled and patients were grouped according to trough (Cmin) concentrations of ≤5ng/mL, >5 and ≤8ng/mL, or >8 ng/mL. Daily dose conversion rates from TacBID to LCPT ranged from 1:0.7 to 1:0.85. PK parameters between drugs were compared at each trough range. Results: Fifty-three, 115, and 68 patients had trough levels in the low, medium, and high ranges, respectively. Patients treated with LCPT consistently showed lower peaks, less fluctuation, and comparable trough levels in each concentration range (Table 1). Correlation between Cmin and AUC was significant for LCPT in all ranges, but was highest (0.83, p<0.001) in the high trough patients. Conclusion: Pooled analysis of 3 PK studies show that conversion from TacBID to LCPT results in consistently reduced peak concentrations, less fluctuation and similar trough levels at reduced daily doses, regardless of trough level range. Data here demonstrate that LCPT maintains its smoother PK profile with once-daily dosing at a variety of clinically applicable trough targets in kidney transplant recipients.
CITATION INFORMATION: Malvezzi P., Bunnapradist S., Stevens D., Moten M., Loh A., Patel S., Rostaing L. Tacrolimus Pharmacokinetics (PK) at Low, Medium, and High Trough Levels in Patients Converted from Twice-Daily Immediate-Release Tacrolimus (TacBID) to Once-Daily Extended-Release Tacrolimus (LCPT) Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Malvezzi P, Bunnapradist S, Stevens D, Moten M, Loh A, Patel S, Rostaing L. Tacrolimus Pharmacokinetics (PK) at Low, Medium, and High Trough Levels in Patients Converted from Twice-Daily Immediate-Release Tacrolimus (TacBID) to Once-Daily Extended-Release Tacrolimus (LCPT) [abstract]. https://atcmeetingabstracts.com/abstract/tacrolimus-pharmacokinetics-pk-at-low-medium-and-high-trough-levels-in-patients-converted-from-twice-daily-immediate-release-tacrolimus-tacbid-to-once-daily-extended-release-tacrolimus-lcpt/. Accessed July 18, 2025.« Back to 2018 American Transplant Congress
