Sustained Biologic Response To Neoadjuvant Therapy Predicts Excellent Liver Transplant Outcomes For Locally Advanced Intrahepatic Cholangiocarcinoma
1J.C. Walter Jr. Transplant Center, Houston Methodist Hospital, Houston, TX, 2The University of Texas MD Anderson Cancer Center, Houston, TX, 3Department of Oncology, Houston Methodist Hospital, Houston, TX
Meeting: 2019 American Transplant Congress
Abstract number: 147
Keywords: Liver, Liver transplantation, Survival, Tumor recurrence
Session Information
Session Name: Concurrent Session: Liver Transplant Oncology
Session Type: Concurrent Session
Date: Sunday, June 2, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:54pm-5:06pm
Presentation Time: 4:54pm-5:06pm
Location: Room 311
*Purpose: Intrahepatic cholangiocarcinoma (iCCA) is a contraindication for liver transplant (LT), but prior studies limited evaluation to incidental iCCA. We recently reported significant improvement in long-term LT outcomes in locally advanced (>5cm) iCCA for pts demonstrating stability >6mo on neoadjuvant chemotherapy (Lunsford 2018 Lancet Gastro Hep). Herein, we report continued pt accrual and long-term follow-up outcomes of the first prospective series evaluating neoadjuvant therapy on LT outcomes for iCCA.
*Methods: Per Methodist-MD Anderson protocol, pts without extrahepatic disease or vascular involvement received gemcitabine/cisplatin-based neoadjuvant therapy. Pts with >6mo stability were listed for LT.
*Results: From 2010-2018, 9pts with iCCA underwent LT. Median time from diagnosis to LT was 26mo (IQR 17-33). Median follow-up was 38mo (IQR 6-51mo). Explant characteristics are shown in Table1.
| Characteristics | Median (IQR) or N (%) |
| Number of lesions | 3 (1-8) |
| Maximum size largest lesion (cm) | 6.5 (4.2-8.5) |
| Cumulative Diameter (cm) | 8.5 (6.5-15.3) |
| Grade | 2 (2-3) |
| Lymphovascular Invasion | 4 (44%) |
| Perineural Invasion | 3 (33%) |
| Multifocal disease | 6 (67%) |
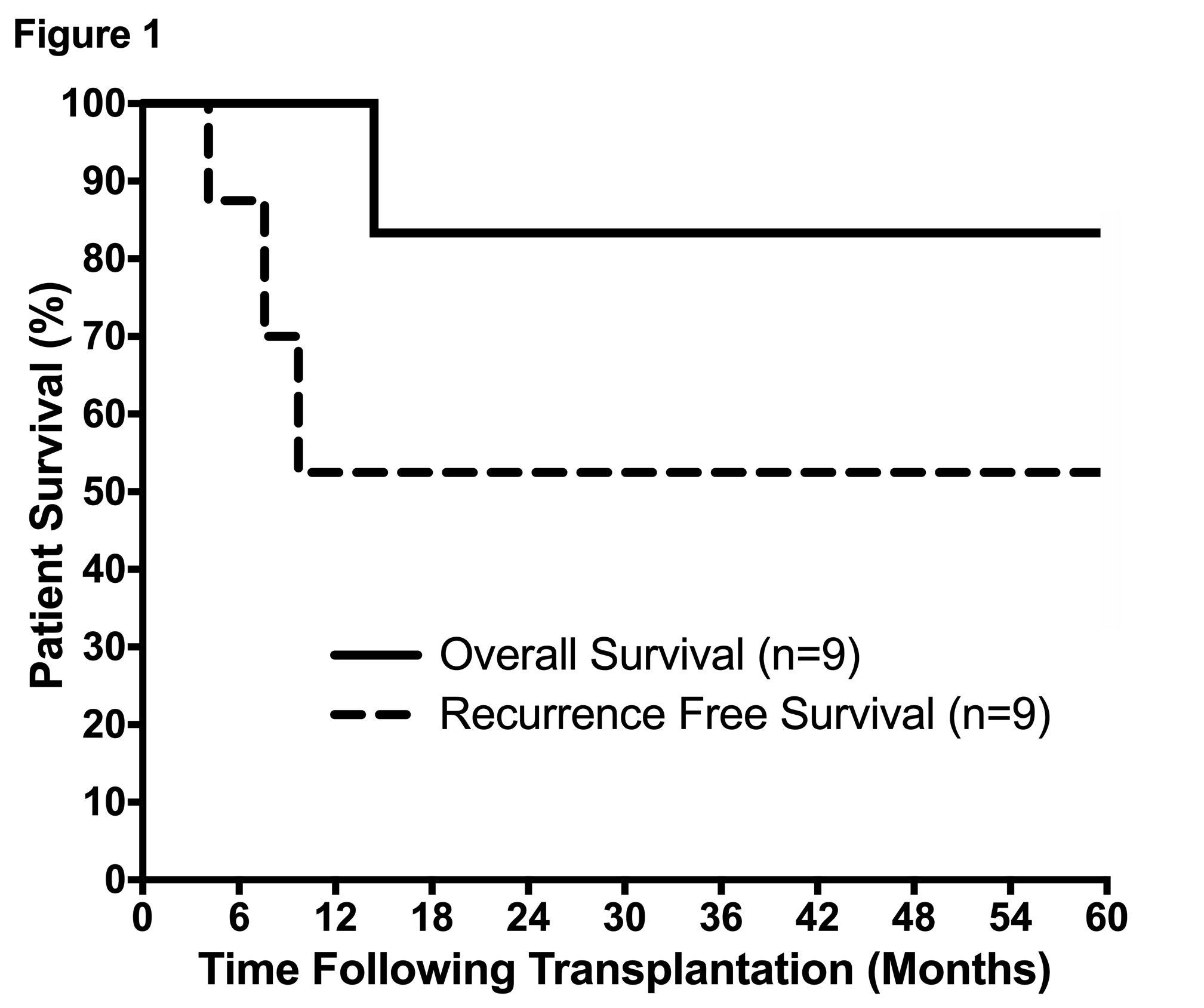
Median cumulative diameter was 8.5cm with median largest lesion 6.5cm. 6/9 (67%) had multifocal disease. Tumors were well differentiated in 1/9, moderately differentiated in 4/9, and poorly differentiated in 4/9. One pt died 14mo post-LT due to recurrence, with overall survival of 100%, 83%, and 83% at 1-,3-&5yrs. To date, 3/9 (33%) pts developed recurrence a median of 7.1mo after LT, with recurrence free survival of 83%, 50%, and 50% at 1-,3-&5yrs (Figure1). There were no discernable associations of recurrence with grade, stage, perineural, or lymphovascular invasion.
*Conclusions: Compared previously reported outcomes, chemosensitivity may select for biologically favorable iCCA for LT, and tumor biology rather than size may dictate iCCA recurrence. Preliminary results suggest iCCA may be acceptable indication for LT in select patients with biologically favorable disease.
To cite this abstract in AMA style:
Lunsford KE, Javle M, Heyne K, Ali RA, Mobley CM, Saharia A, Victor DW, Hobeika MJ, Kasab A, McFadden RS, Aloia TA, Li XC, Monsour HP, Gaber AO, Gaber AO, Vauthey J, Ghobrial RM. Sustained Biologic Response To Neoadjuvant Therapy Predicts Excellent Liver Transplant Outcomes For Locally Advanced Intrahepatic Cholangiocarcinoma [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/sustained-biologic-response-to-neoadjuvant-therapy-predicts-excellent-liver-transplant-outcomes-for-locally-advanced-intrahepatic-cholangiocarcinoma/. Accessed February 16, 2026.« Back to 2019 American Transplant Congress