Steroid Free Immunosuppression Protocol in Pediatric Kidney Transplant Recipients Using Alemtuzumab: A Retrospective Single Center Study
1Surgery, Hershey Medical Center, Hershey, PA
2Pediatric Nephrology, Hershey Medical Center, Hershey, PA.
Meeting: 2015 American Transplant Congress
Abstract number: 131
Keywords: Antilymphocyte antibodies, Kidney transplantation, Pediatric, Post-transplant lymphoproliferative disorder (PTLD)
Session Information
Session Name: Concurrent Session: Pediatric Kidney Transplantation
Session Type: Concurrent Session
Date: Sunday, May 3, 2015
Session Time: 4:00pm-5:30pm
 Presentation Time: 5:12pm-5:24pm
Presentation Time: 5:12pm-5:24pm
Location: Room 119-A
Background: Steroid free immunosuppression is attractive in pediatric kidney transplantation (KTx). Aim of the present study is to examine the patient survival, graft survival, rate of cellular and humoral rejection, and the side effects.
Patients and Method: 62 consecutive children received KTx between 7/2005 to 9/ 2013 (mean age 10.5, 34 male, 26 live donors, and 10 were second KTx). All patients were followed up to Sept.2014. (Mean follow-up 5.7 yr.). All children received, 0.35 mg/kg intravenous Alemtuzumab over two hours prior to reperfusion with premedication. Post operatively, tacrolimus was administered 0.05 to 0.1mg/kg Q12h after 24 hours to achieve trough concentrations of 8 to 12 ng/mL. Mycophenolate Mofetil was started at 150mg/meter square BSA Q12h and. corticosteroids were not used.
Results: Patient Survival: One child died from PTLD after 2 years. One year patient survival was 100% and 98.4% at seven year. Graft survival: Total Eight grafts were lost of which two were in first year. Kaplan Meier survival was 95.1 % at year and 83% at 7 year.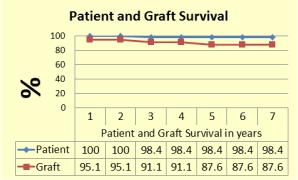 Rate of Rejection: 343 renal biopsies were performed as clinically indicated. 21patients (33.9%) experienced cellular rejection in the first year post KTx .In addition 8 children experienced cellular rejection from 1 to 9 years of follow. Eleven children experienced C4d stain positive humoral rejection in first year post KTx; two of these were without cellular rejection. Overall freedom from rejection in first year was 62.9%. Leukopenia with absolute neutrophils count of <1000 was noted in 47 (75.8%) children which was managed with granulocyte colony stimulating factor. Three children suffered from PTLD.
Rate of Rejection: 343 renal biopsies were performed as clinically indicated. 21patients (33.9%) experienced cellular rejection in the first year post KTx .In addition 8 children experienced cellular rejection from 1 to 9 years of follow. Eleven children experienced C4d stain positive humoral rejection in first year post KTx; two of these were without cellular rejection. Overall freedom from rejection in first year was 62.9%. Leukopenia with absolute neutrophils count of <1000 was noted in 47 (75.8%) children which was managed with granulocyte colony stimulating factor. Three children suffered from PTLD.
Conclusion: In our experience, Alemtuzumab provides steroid free immunosuppression in pediatric KTx recipients, with one year patient survival and graft survival of 100% and 95.1% and 98.4% and 87.6% at 7 year, respectively. First year freedom from rejection was 62.9%. Leukopenia occurred in 76% of cases with rate of PTLD of 4%.
To cite this abstract in AMA style:
Shah R, Kadry Z, Wassner S, Kees-Folts D, Freeman M, Freeman P, Olenowski B, Jain A. Steroid Free Immunosuppression Protocol in Pediatric Kidney Transplant Recipients Using Alemtuzumab: A Retrospective Single Center Study [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/steroid-free-immunosuppression-protocol-in-pediatric-kidney-transplant-recipients-using-alemtuzumab-a-retrospective-single-center-study/. Accessed February 24, 2026.« Back to 2015 American Transplant Congress
