Safety and Efficacy of Endoscopic Transpapillary Gallbladder Stenting for Symptomatic Gallbladder Disease in Cirrhosis: A Systematic Review and Meta-Analysis
K. F. Haq1, U. Iqbal2, M. Khan3, F. Kamal4, S. Solanki5, R. Chakinala5, M. Siddiqui1, M. Abu Ghanimeh1, O. Sadiq1, A. Watson1, T. Zuchelli1, R. Salgia1
1Gastroenterology & Hepatology, Henry Ford Hospital, Detroit, MI, 2Medicine, Geisinger Medical Center, Danville, PA, 3Gastroenterology & Hepatology, University of Alabama, Birmingham, AL, 4Gastroenterology & Hepatology, University of Tennessee, Knoxville, TN, 5Medicine, Guthrie Hospital, Sayre, PA
Meeting: 2020 American Transplant Congress
Abstract number: A-109
Keywords: Efficacy, Liver cirrhosis, Meta-analysis, Safety
Session Information
Session Name: Poster Session A: Liver: Portal Hypertension and Other Complications of Cirrhosis
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Endoscopic transpapillary gallbladder (cystic duct) stenting has been shown to be an effective treatment option for symptomatic gallbladder disease in patients with cirrhosis. However, evidence is limited at this time.
*Methods: We searched databases including Embase, Cochrane Central, and PubMed from inception to August 2019 to identify all studies that evaluated outcomes of endoscopic transpapillary gallbladder stenting (ETGS) for symptomatic gallbladder disease in patients with cirrhosis. Primary outcome was technical success defined as successful stenting and clinical success defined as resolution of symptoms. Pooled estimates and measures of variability from studies were used to generate forest plots. Publication bias was evaluated by Egger’s test. Variability between studies was assessed by heterogeneity tests using I2 statistics. All analyses were conducted using RStudio (Version 1.0.136) using the ‘Meta’ and ‘Metafor’ package.
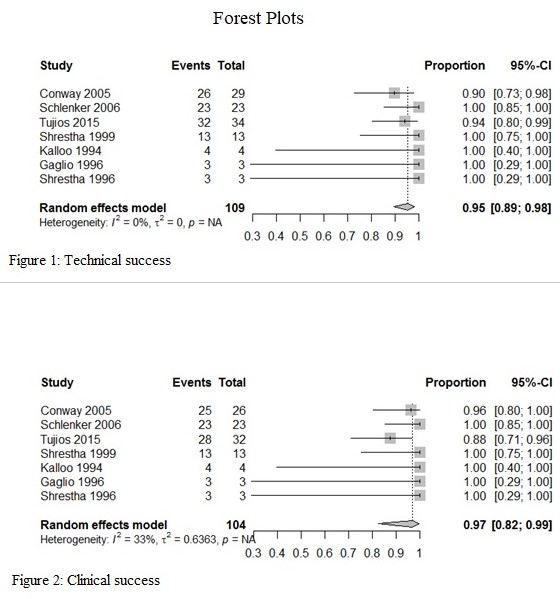
*Results: Seven studies including 109 patients met our inclusion criteria. At least, 94 patients (86%) had a Child-Pugh score of B or C. Most common indications for ETGS were recurrent biliary colic (51.3%), acute cholecystitis (33.9%), and biliary pancreatitis (11.9%). ETGS was technically successful in 104 patients with pooled technical success of 95% (95% CI: 89%-98%) with no statistical heterogeneity as calculated by I2=0. In patients with successful ETGS, clinical success was achieved in 99 patients with pooled clinical success of 97% (95% CI: 82%-99%) with statistical heterogeneity I2=33%. Adverse events were reported in 15.6%, most commonly pancreatitis (4.6%), cholangitis (3.7%), and duodenal ulceration (1.8%). Technical failure was due to inability to cannulate cystic duct (1.8%), impacted cystic duct stone (1.8%), and tortuous cystic duct complicated by perforation requiring cholecystostomy tube (0.9%). Overall mortality was less than 1% as one patient with acute cholecystitis ultimately required open cholecystectomy that was complicated by sepsis and death.
*Conclusions: ETGS is a relatively safe and effective therapeutic modality for symptomatic gallbladder disease in patients with end-stage liver disease.
To cite this abstract in AMA style:
Haq KF, Iqbal U, Khan M, Kamal F, Solanki S, Chakinala R, Siddiqui M, Ghanimeh MAbu, Sadiq O, Watson A, Zuchelli T, Salgia R. Safety and Efficacy of Endoscopic Transpapillary Gallbladder Stenting for Symptomatic Gallbladder Disease in Cirrhosis: A Systematic Review and Meta-Analysis [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-endoscopic-transpapillary-gallbladder-stenting-for-symptomatic-gallbladder-disease-in-cirrhosis-a-systematic-review-and-meta-analysis/. Accessed February 25, 2026.« Back to 2020 American Transplant Congress