Safety and Efficacy of a Probiotic Protocol to Reduce Perioperative Infection After Liver Transplantation
M. Jorgenson, Q. Yang, G. Leverson, C. Saddler, J. Smith, N. Safdar, D. Al-Adra
University of Wisconsin Health, Madison, WI
Meeting: 2022 American Transplant Congress
Abstract number: 697
Keywords: Graft survival, Infection, Liver transplantation, Rejection
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) I
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: Evaluate efficacy and safety of a probiotic preparation to reduce perioperative infection in patients undergoing liver transplant surgery
*Methods: Adult patients receiving liver transplantation from 5/1/2019-9/30/2020 received a probiotic preparation of lactobacillus+guar gum prebiotic fibers given perioperatively for 14 days (POD 0-POD 14) and were compared to a standard of care (SOC) cohort who received liver transplant in the year prior to implementation of the protocol (3/1/2018-3/1/2019). All patients received perioperative and transplant infection prophylaxis per protocol. Primary objective was incidence of bacterial infection within 30 days of transplant. Secondary objectives were safety/toxicity as measured by lactobacillus-associated infectious events and rates of 1 year biopsy proven acute rejection (BPAR), graft and patient survival
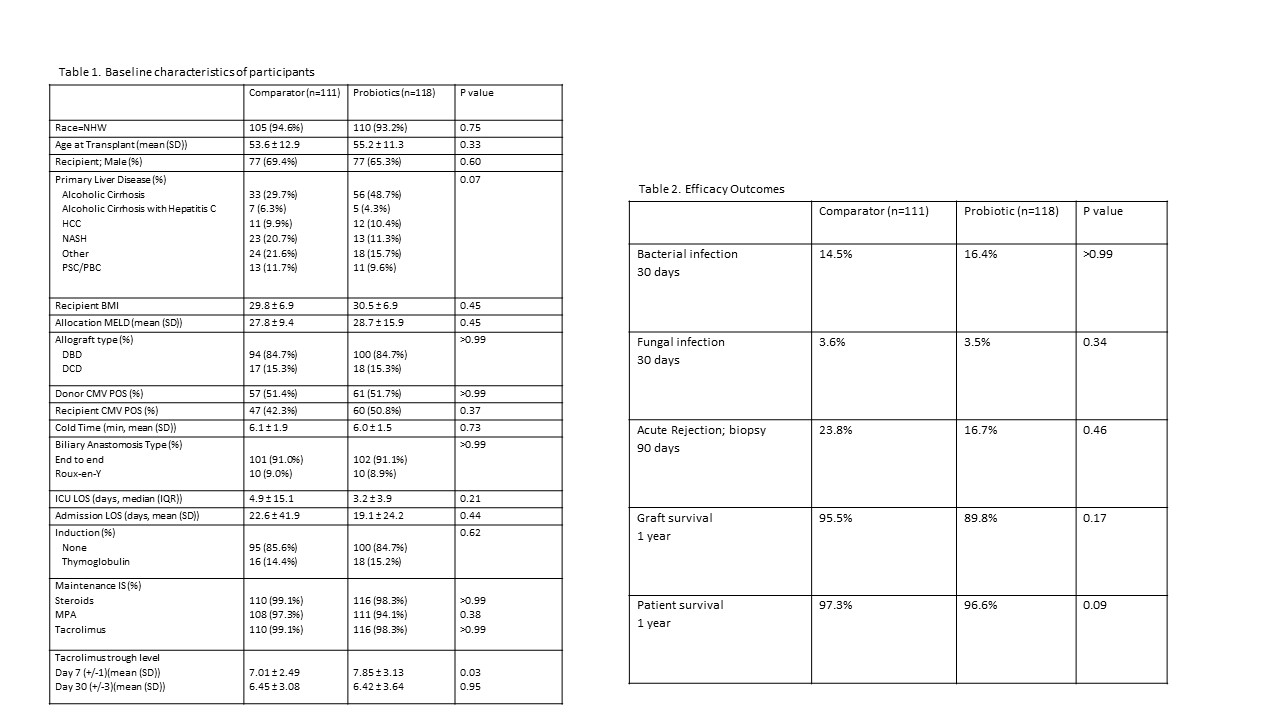
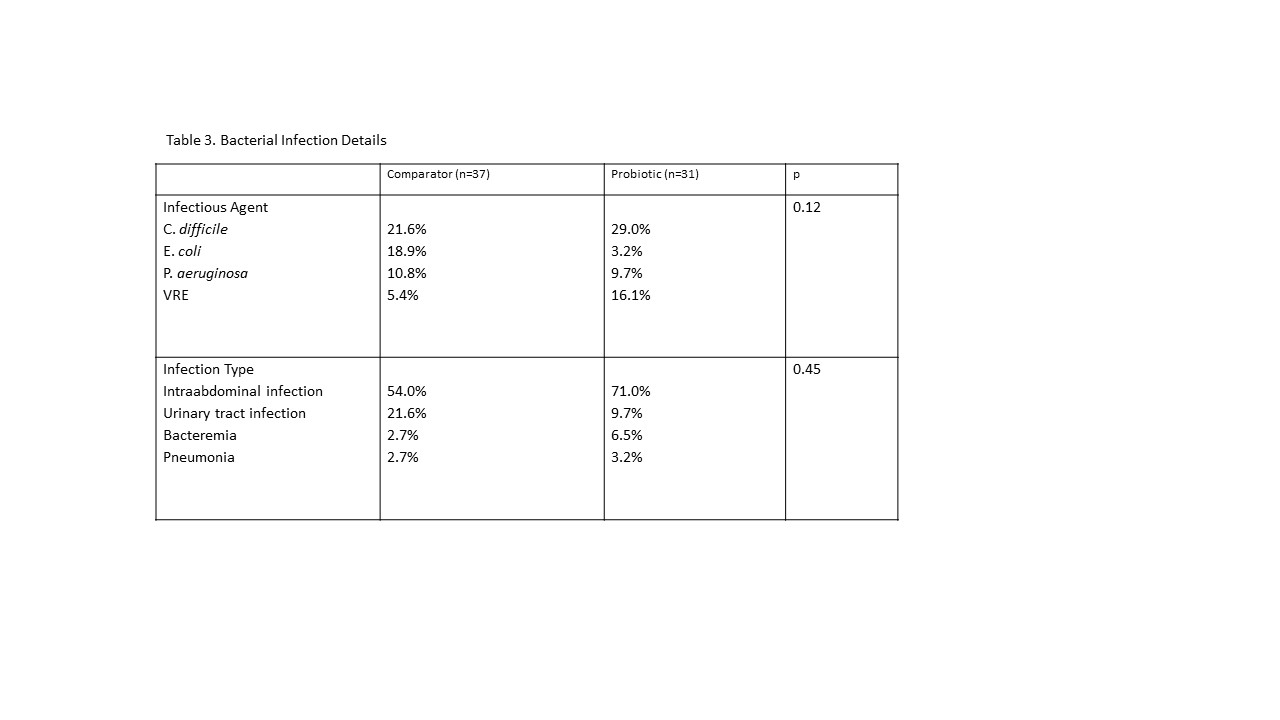
*Results: 229 patients met inclusion criteria; 118 in the probiotic cohort, 111 in the SOC cohort. Clinical characteristics were similar between groups (Table 1). Patients who received the probiotic preparation had a similar incidence of perioperative bacterial infective events (probiotic 14.5% vs SOC 16.4%, p>0.99, Table 2). There was no significant change in bacterial epidemiology or infection type pre-to post-intervention. (Table 3) There were no lactobacillus-associated infectious events in the probiotic cohort (probiotic 0% vs SOC 1.8%, p>0.99). Rates of BPAR (23.8% vs 16.7%, p=0.46), graft (95.5% vs 89.8%, p=0.17) and patient survival (97.3% vs 96.6%, p=0.09) were not different. In a subgroup analysis of patients transplanted for alcoholic cirrhosis, presumed to have the highest degree of dysbiosis, efficacy outcomes were also not significantly different (bacterial infection: 12.9% vs 14.9%, p=0.45, BPAR: 20.6% vs 18.0%, p=0.42, graft survival: 95.0% vs 96.7%, p=0.53).
*Conclusions: Examination of the probiotic protocol suggests safety based on lack of lactobacillus-associated infections in the probiotic group. However, the protocol did not appear to have a significant impact on efficacy outcomes in the current era of liver transplantation, although numerically reduced rates of BPAR may warrant further investigation.
To cite this abstract in AMA style:
Jorgenson M, Yang Q, Leverson G, Saddler C, Smith J, Safdar N, Al-Adra D. Safety and Efficacy of a Probiotic Protocol to Reduce Perioperative Infection After Liver Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-a-probiotic-protocol-to-reduce-perioperative-infection-after-liver-transplantation/. Accessed March 3, 2026.« Back to 2022 American Transplant Congress