Risk of Post-Transplant Lymphoproliferative Disease After Anti-Thymocyte Globulin in Childhood Solid-Organ Transplant Recipients
1Transplant & Regenerative Medicine Centre, The Hospital for Sick Children, Toronto, ON, Canada, 2Child Health Evaluative Sciences, The Hospital for Sick Children, Toronto, ON, Canada, 3Division of Nephrology, Department of Medicine, Women’s College Hospital and University Health Network, Toronto, ON, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 61
Keywords: Antilymphocyte antibodies, Immunosuppression, Malignancy, Pediatric
Topic: Clinical Science » Infection Disease » 28 - PTLD: All Topics
Session Information
Session Name: PTLD and Malignancies
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:50pm-4:00pm
Presentation Time: 3:50pm-4:00pm
Location: Hynes Room 309
*Purpose: Determine if the use of anti-thymocyte globulin (ATG) in pediatric solid organ transplant recipients was associated with an increased risk of post-transplant lymphoproliferative disorder (PTLD) in a dose-response dependent manner.
*Methods: In a retrospective study of solid organ transplant recipients < 18 years of age at a large pediatric transplant centre, between January 1, 2002 to December 31, 2015 with a minimum follow up period of 14 days, we assessed the association of ATG cumulative dose (tertiles) with the risk of PTLD by Cox regression. All PTLD classifications were included (florid follicular hyperplasia, plasmacytic hyperplasia, infectious mononucleosis, polymorphic, monomorphic and Hodgkin lymphoma PTLD).
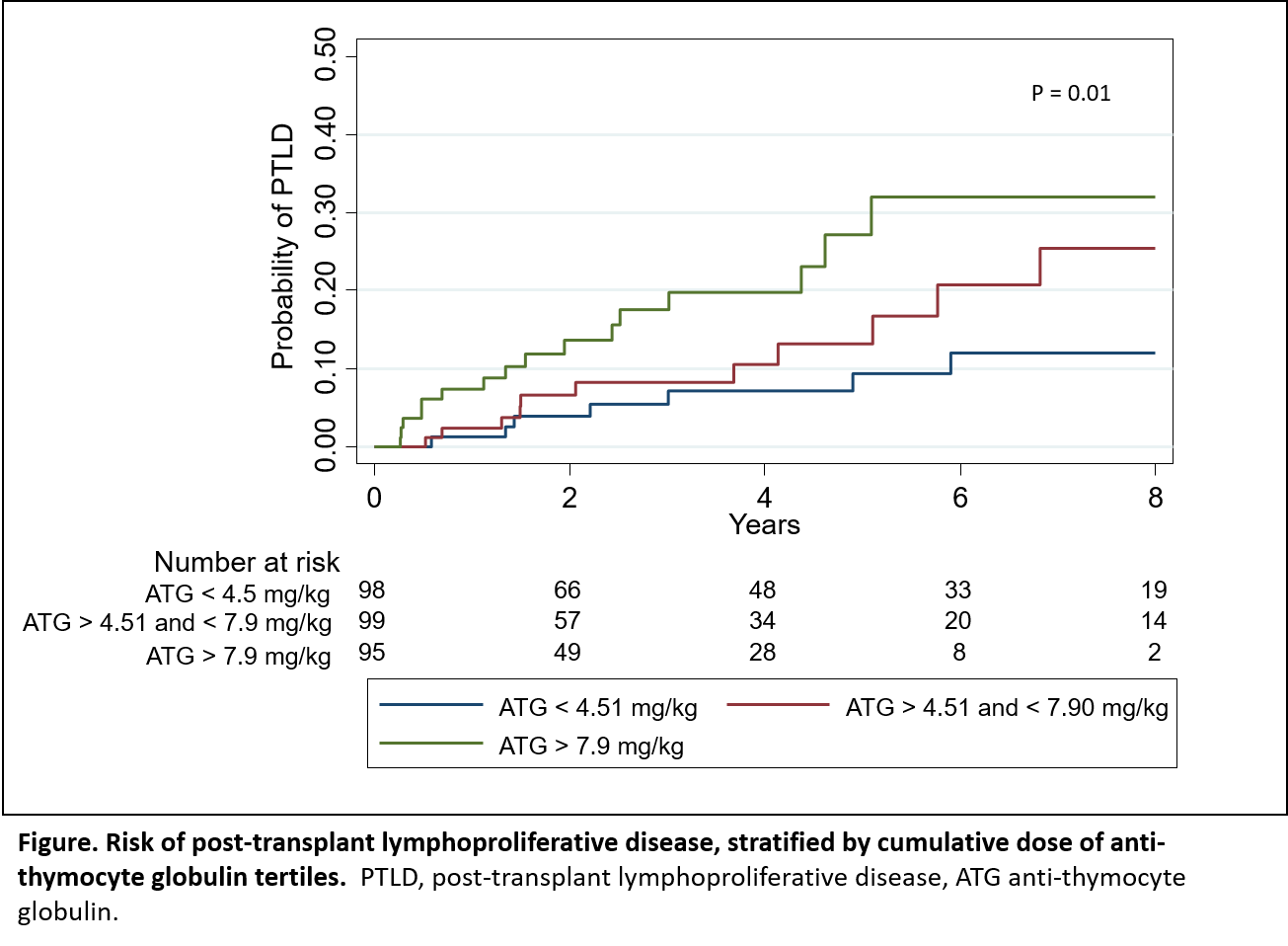
*Results: A total of 611 pediatric solid organ transplant recipients (liver, kidney, heart and lung) were included in the study. Median age at transplant was 6.1 yrs. (IQR 1.01 to 13.39) and 53% were male. Thirty-eight percent (234) underwent liver, 29% (177) kidney, 28% (168) heart and 5% (32) lung transplantation. Forty-eight percent (n=292) received ATG for induction or treatment of rejection during the follow up period with a median cumulative dose of 5.9mg/kg (IQR 3.64 to 8.96). Those who received ATG were more likely to be heart or kidney transplant recipients and receive a deceased donor organ (P<0.001). PTLD occurred in 81 children (13%), with a median time to diagnosis of 1.5 years (IQR 0.53 to 3.32). Among transplant recipients who received ATG, incidence of PTLD increased with increasing cumulative ATG dose (Figure). Cumulative ATG dose (mg/kg), age at transplant and organ type were associated with risk of PTLD. For every 10% increase in ATG cumulative dose, the risk of PTLD increased by 7%, when adjusted for age at transplant, sex, donor type and organ type (log (cumulative ATG dose) HR 2.0; 95% CI 1.16 to 3.50, P < 0.01).
*Conclusions: Among pediatric solid organ transplant recipients who received ATG, the risk of PTLD increased significantly in a dose dependent manner by cumulative ATG dose.
To cite this abstract in AMA style:
McKay AM, Teoh C, Vasilevska-Ristovka J, Browne J, Banh T, Dipchand A, Ng V, Solomon M, Allen U, Avitzur Y, Parekh R. Risk of Post-Transplant Lymphoproliferative Disease After Anti-Thymocyte Globulin in Childhood Solid-Organ Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/risk-of-post-transplant-lymphoproliferative-disease-after-anti-thymocyte-globulin-in-childhood-solid-organ-transplant-recipients/. Accessed March 4, 2026.« Back to 2022 American Transplant Congress