Remote Monitoring Using Mobile Phlebotomy and Donor-derived Cell-free DNA in Kidney Transplant Recipients During the Covid-19 Pandemic
1NYU Langone, New York, NY, 2Medical Science Liason, CareDx, Brisbane, CA
Meeting: 2021 American Transplant Congress
Abstract number: 627
Keywords: Genomic markers, Immunosuppression, Kidney transplantation, Monitoring
Topic: Clinical Science » Biomarkers, Immune Assessment and Clinical Outcomes
Session Information
Session Name: Biomarkers, Immune Assessment and Clinical Outcomes
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: The rapid shift to telemedicine and remote monitoring of kidney transplant recipients (KTRs) during COVID-19 aimed to mitigate exposure risk for this vulnerable population. dd-cfDNA (AlloSure, CareDx Brisbane) is a well-established biomarker for surveillance of KTRs and is associated with allograft tissue injury, including immunological events such as acute rejection. Here we describe an innovative approach to remote surveillance for KTRs using mobile home phlebotomy during the pandemic.
*Methods: Pilot program of KTRs enrolled into the mobile home phlebotomy (RemoTraC) from March – November 2020. AlloSure dd-cfDNA was concomitantly performed with routine post-transplant laboratory studies at regular time intervals as per standard of care.
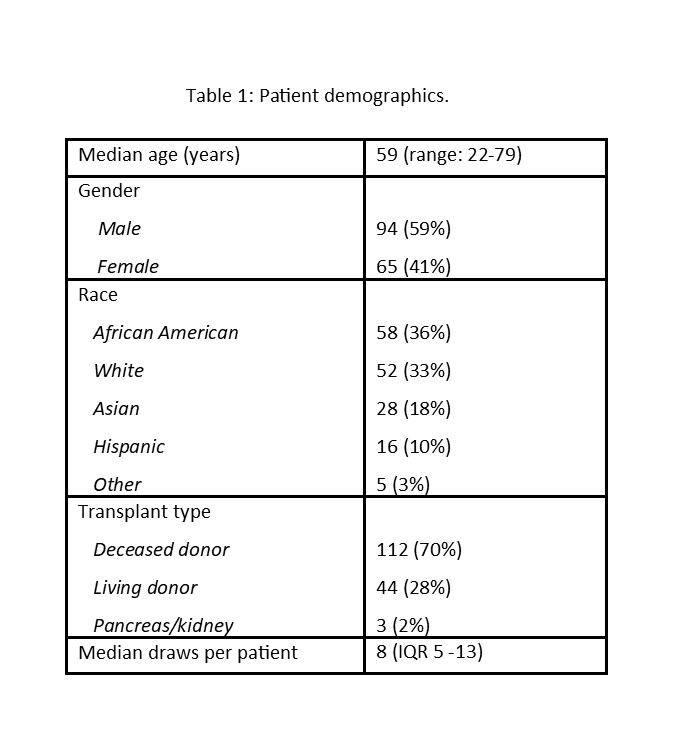
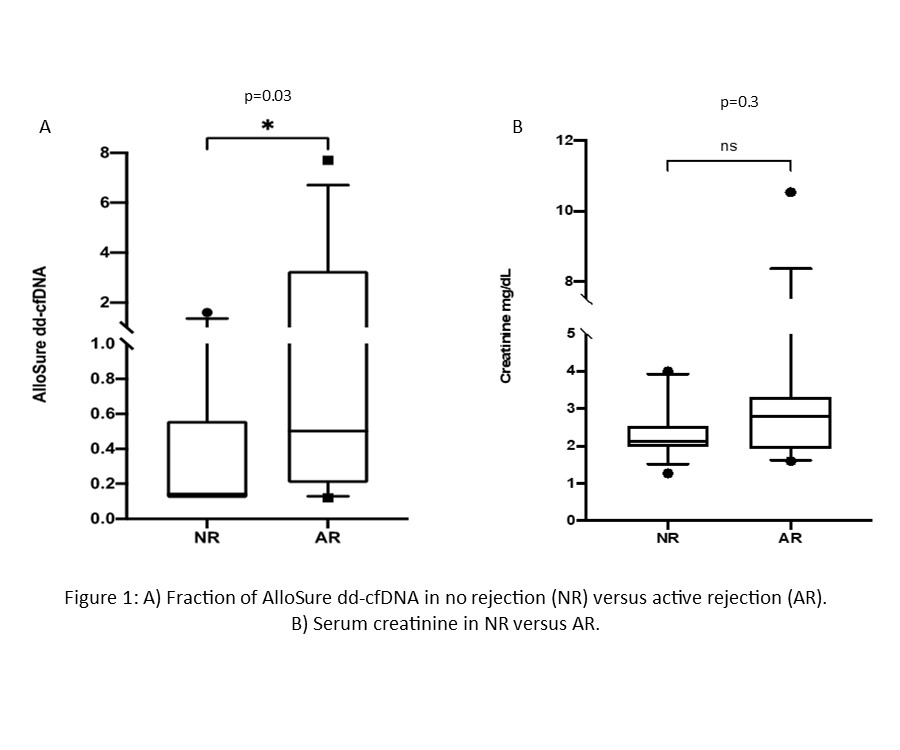
*Results: 159 KTRs were enrolled in the mobile phlebotomy program with 1421 draws completed during the surveillance period. Patient demographics are summarized in Table 1. The median AlloSure dd-cfDNA level was 0.21% (IQR 0.12 – 0.42%). 25 for-cause biopsies were performed in patients monitored with mobile phlebotomy. 12 patients had biopsy proven rejections paired with AlloSure dd-cfDNA (1 borderline, 2 TCMR1A, 4 TCMR2A, 2 TCMR1B, 2 chronic active TCMR and 1 mixed AMR/TCMR). The median AlloSure dd-cfDNA was 0.5% (IQR 0.2-3.26%) in patients with active rejection compared to 0.14% (IQR 0.12-0.56%) in patients with no rejection (p=0.03). There was no difference in serum creatinine between the two groups (p=0.3). The median AlloSure dd-cfDNA levels in TCMR2A/1B and mixed AMR/TCR were 0.72 and 7.7% respectively.
*Conclusions: AlloSure dd-cfDNA can optimize post-transplant care by identifying patients at risk of allograft injury and rejection. This analysis demonstrates the feasibility of mobile phlebotomy for routine surveillance in combination with telehealth strategies during the unprecedented COVID-19 pandemic. In addition, utilization of dd-cfDNA helped clinicians direct limited resources during the pandemic for allograft biopsies when paired with standard clinical markers such as creatinine.
To cite this abstract in AMA style:
Ali N, Miles J, Tatapudi V, Montgomery R. Remote Monitoring Using Mobile Phlebotomy and Donor-derived Cell-free DNA in Kidney Transplant Recipients During the Covid-19 Pandemic [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/remote-monitoring-using-mobile-phlebotomy-and-donor-derived-cell-free-dna-in-kidney-transplant-recipients-during-the-covid-19-pandemic/. Accessed March 1, 2026.« Back to 2021 American Transplant Congress