Recipient Race Modifies the Association Between Obesity and Long Term Graft Outcomes After Renal Transplantation
1Dalhousie University, Halifax, NS, Canada, 2Nova Scotia Health Authority, Halifax, NS, Canada, 3Nova Scotia Health Authority Division of Nephrology, Departm, Halifax, NS, Canada
Meeting: 2022 American Transplant Congress
Abstract number: 305
Keywords: African-American, Graft failure, Graft survival, Obesity
Topic: Clinical Science » Kidney » 46 - Kidney Complications: Non-Immune Mediated Late Graft Failure
Session Information
Session Name: Kidney Complications: Non-Immune Mediated Late Graft Failure
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 6, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 6:50pm-7:00pm
Presentation Time: 6:50pm-7:00pm
Location: Hynes Room 310
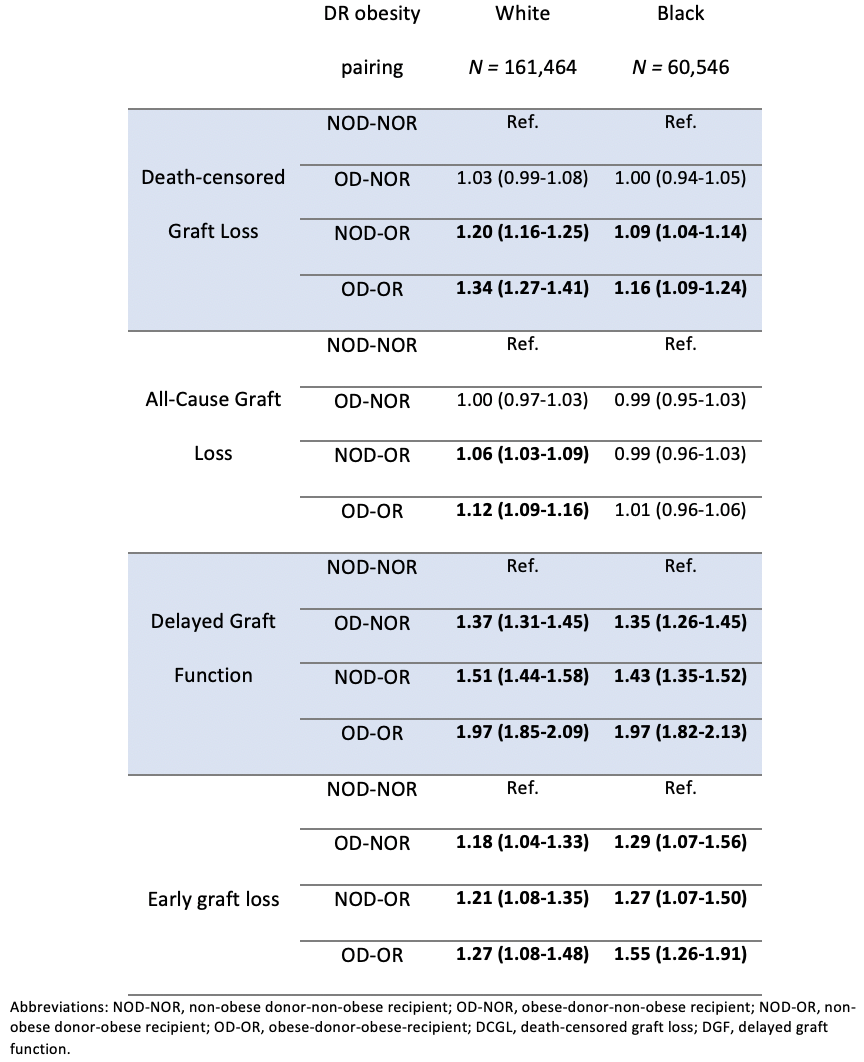
*Purpose: Both donor and recipient obesity are associated with adverse outcomes after transplant. Whether obesity, defined using body mass index (BMI), has different implications on graft outcomes in Black and White recipients is unknown. Here, we explore the association between donor and recipient obesity status and graft outcomes separately in Black and White recipients.
*Methods: We used the SRTR to identify recipients of a kidney transplant (live or deceased) from Jan 2000-Dec 2016. Recipients were stratified by race (White or Black). We dichotomized donor-recipient (DR) obesity status at a BMI cut point of 30 kg/m2 to identify DR obesity pairings: i. non-obese DR (NOD-NOR), ii. obese donor-non obese recipient (OD-NOR), iii. non obese donor-obese recipient (NOD-OR), and iv. obese DR (OD-OR). For each DR obesity pairing, we used multivariable Cox proportional hazards models to determine the hazard for death-censored graft loss (DCGL) and all-cause graft loss, and multivariable logistic regression to determine the odds ratio for delayed graft function (DGF) and early (≤30 day) graft loss (EGL). Finally, we determined if recipient race modified the association between recipient obesity status and each outcome.
*Results: 238,892 transplant recipients were included in our analysis; 67.6% were White and 25.3% Black. DCGL occurred in 12.6%, all-cause graft loss in 34.9%, DGF in 18.1% and early graft loss in 2%. Mean donor and recipient BMI were 27.1±6.0 and 27.7±5.6 kg/m2, respectively. In White recipients, the adjusted hazard for DCGL was highest in the setting of an obese recipient (OR-OR or NOD-OR compared with NOD-NOR). That risk persisted but was attenuated in Black recipients (Table 1). The same trend existed for all-cause graft loss in White, but not Black recipients. DR obesity was similarly associated with risk of DGF and EGL for both White and Black recipients. Black race modified the effect between recipient obesity and both DCGL (p<0.001) and all-cause graft loss (p<0.001), but not DGF (p=0.187) or EGL (p=0.353).
*Conclusions: Our findings indicate that DR obesity, defined using BMI, differentially impacts long-term outcomes in Black and White recipients. BMI may not reflect increased risk of poor long-term outcomes in Black patients after kidney transplant. This finding is supported by previous studies which have demonstrated differences in the body composition of Black and White individuals, which make BMI more likely to overestimate adiposity in Black patients.
To cite this abstract in AMA style:
Jarrar F, Tennankore K, Vinson AJ. Recipient Race Modifies the Association Between Obesity and Long Term Graft Outcomes After Renal Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/recipient-race-modifies-the-association-between-obesity-and-long-term-graft-outcomes-after-renal-transplantation/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress