Randomized Phase 3 Open-label Study of Maribavir vs Investigator-assigned Therapy for Refractory/resistant Cytomegalovirus Infection in Transplant Recipients: Subgroup Analyses of Efficacy by Organ
R. K. Avery1, E. A. Blumberg2, D. Florescu3, N. Kamar4, D. Kumar5, J. Wu6, A. Sundberg6
1Johns Hopkins Hospital, Baltimore, MD, 2University of Pennsylvania, Philadelphia, PA, 3University of Nebraska School of Medicine, Omaha, NE, 4Hôpital de Rangueil, Toulouse, France, 5University Health Network, Toronto, ON, Canada, 6Shire Human Genetic Therapies, Inc., a Takeda company, Lexington, KY
Meeting: 2021 American Transplant Congress
Abstract number: LB 9
Keywords: Cytomeglovirus, Heart transplant patients, Kidney transplantation, Lung transplantation
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: Late Breaking: Basic & ID
Session Type: Rapid Fire Oral Abstract
Date: Monday, June 7, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-6:05pm
Presentation Time: 6:00pm-6:05pm
Location: Virtual
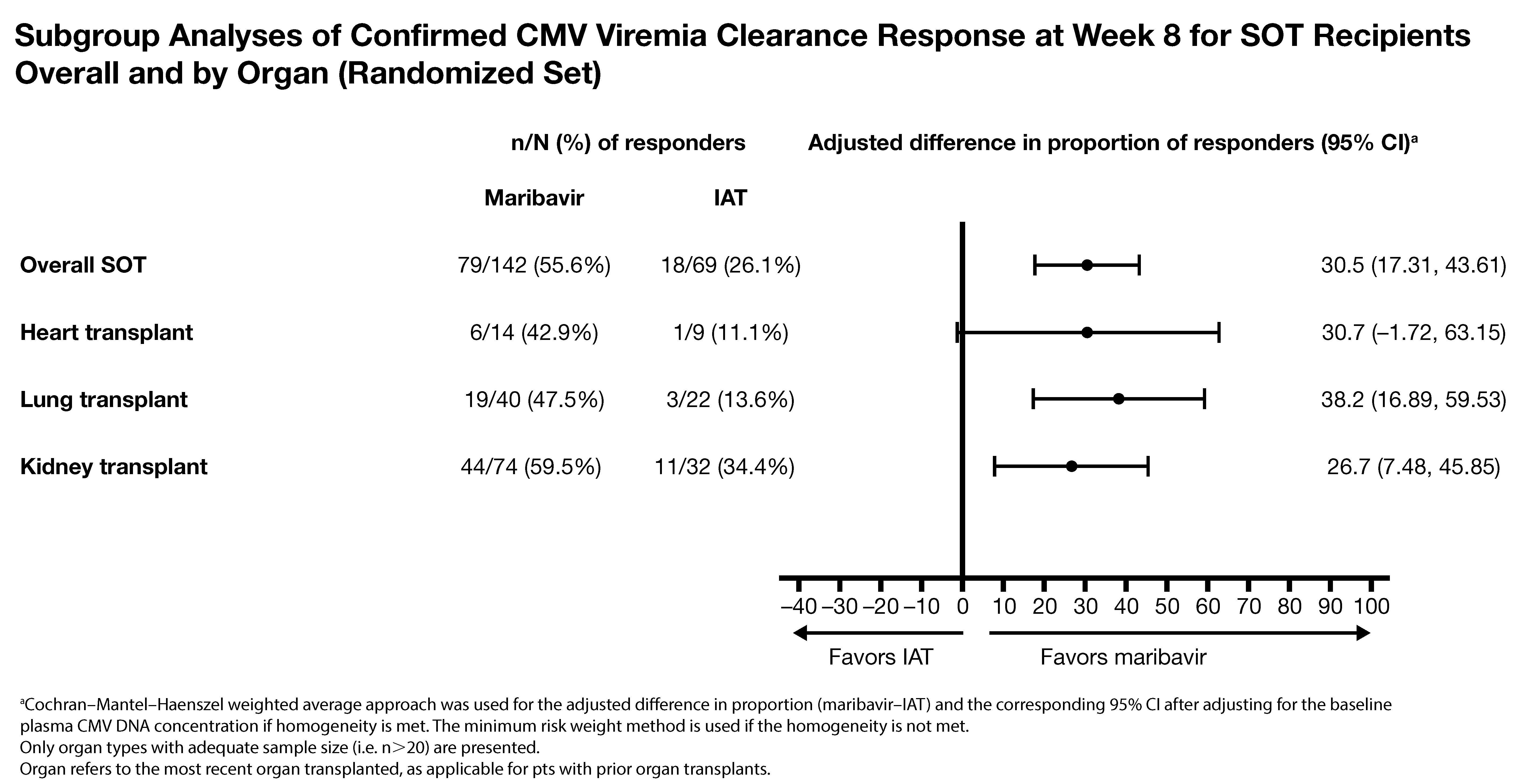
*Purpose: Therapeutic options for refractory, with/without resistance (R/R), CMV infections are limited. We report organ type subgroup analyses from a large multi-center trial that studied the efficacy of maribavir (MBV) vs investigator-assigned therapy (IAT) in pts with R/R CMV infection.
*Methods: Transplant recipients aged ≥12 yrs, with CMV infection (viral load [VL] ≥2730 IU/mL/≥910 IU/mL CMV DNA [blood/plasma]) refractory to recent Tx (failure to achieve >1 log10 decrease in CMV DNA after ≥14 days) were eligible (NCT02931539). Pts were stratified (HCT/SOT + screening CMV VL) and randomized 2:1 to MBV 400 mg BID or IAT (val/ganciclovir, foscarnet, cidofovir, foscarnet+val/ganciclovir) for 8 wks + 12 wks follow-up. Primary endpoint: confirmed CMV clearance (plasma CMV DNA <137 IU/mL in 2 consecutive tests ≥5 days apart) at end of Wk 8. Key secondary endpoint: CMV clearance and symptom control at end of Wk 8 and maintained through Wk 16. Group differences, adjusted for baseline CMV DNA level <9100/≥9100 IU/mL, and SOT/HCT were compared (Cochran-Mantel-Haenszel tests). Subgroup analyses by SOT type were conducted.
*Results: 352 pts were randomized (235 MBV, 117 IAT; age range 19-79 years). Significantly more pts (MBV vs IAT) achieved the primary (55.7% vs 23.9%; difference, 95% CI: 32.8%, 22.8-42.7; p<0.001) and key secondary endpoint (18.7% vs 10.3%; difference, 95% CI: 9.5%, 2.0-16.9; p=0.013). 211 pts (59.9%) were SOT recipients (kidney, 50.2%; lung, 29.4%; heart, 10.9%; liver, 3.3%; pancreas, 0.9%; intestine, 0.5%; multiple, 4.7%). A benefit trend for MBV vs IAT in kidney, lung, and heart transplants was seen (Fig). No SOT pts lost grafts. Tx-emergent AEs (TEAEs) with MBV vs IAT (overall % pts): 97.4% and 91.4%. Acute kidney injury with MBV vs foscarnet was lower: 8.5% vs 21.3% (TEAE) and 1.7% vs 19.1% (Tx-related TEAE). Neutropenia with MBV vs val/ganciclovir was lower: 9.4% vs 33.9% (TEAE) and 1.7% vs 25.0% (Tx-related TEAE). Overall, 2 Tx-related serious TEAEs led to death (1 pt per arm).
*Conclusions: MBV showed superior efficacy vs IAT in clearing CMV in transplant recipients with R/R CMV infection, with consistent trends across organ types and lower rates of Tx limiting toxicities common with IAT.
To cite this abstract in AMA style:
Avery RK, Blumberg EA, Florescu D, Kamar N, Kumar D, Wu J, Sundberg A. Randomized Phase 3 Open-label Study of Maribavir vs Investigator-assigned Therapy for Refractory/resistant Cytomegalovirus Infection in Transplant Recipients: Subgroup Analyses of Efficacy by Organ [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/randomized-phase-3-open-label-study-of-maribavir-vs-investigator-assigned-therapy-for-refractory-resistant-cytomegalovirus-infection-in-transplant-recipients-subgroup-analyses-of-efficacy-by-organ/. Accessed February 19, 2026.« Back to 2021 American Transplant Congress