Prospective, Randomized Study of Conversion from Calcineurin Inhibitor (CNI)- to Belatacept (BELA)-Based Maintenance Immunosuppression in Renal Transplant Recipients
1Charité Universitätsmedizin Berlin, Berlin, Germany, 2Henry Ford Hospital, Detroit, MI, 3The Hannover Medical School, Hannover, Germany, 4Instituto de Nefrologia, Nephrology SA, Buenos Aires, Argentina, 5University of Toulouse, Toulouse, France, 6UVA Health, Charlottesville, VA, 7Leiden University Medical Center, Leiden, Netherlands, 8Université Grenoble Alpes, Saint-Martin-d'Hères, France, 9University Medical Centre Groningen, Groningen, Netherlands, 10University of Wisconsin, Madison, WI, 11UW Medical Center, Seattle, WA, 12Bristol-Myers Squibb, Princeton, NJ, 13University of California, San Francisco, San Francisco, CA
Meeting: 2020 American Transplant Congress
Abstract number: 112
Keywords: Calcineurin, Graft survival, Immunosuppression, Kidney transplantation
Session Information
Session Name: Novel Immunosuppression: Belatacept
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:39pm-3:51pm
Presentation Time: 3:39pm-3:51pm
Location: Virtual
*Purpose: CNIs are associated with adverse systemic and nephrotoxic effects that negatively impact long-term patient and graft survival. In a phase 2 study, switching renal transplant recipients (6-36 months post-transplant) from CNI-based to BELA-based treatment did not increase the risk of death or graft loss.
*Methods: In a phase 3b study (NCT01820572), 446 recipients of living or deceased donor kidneys were randomized (1:1) at 6-60 months post-transplant to convert to BELA or remain on CNI for 24 months. The primary endpoint was death or graft loss at Month 24.
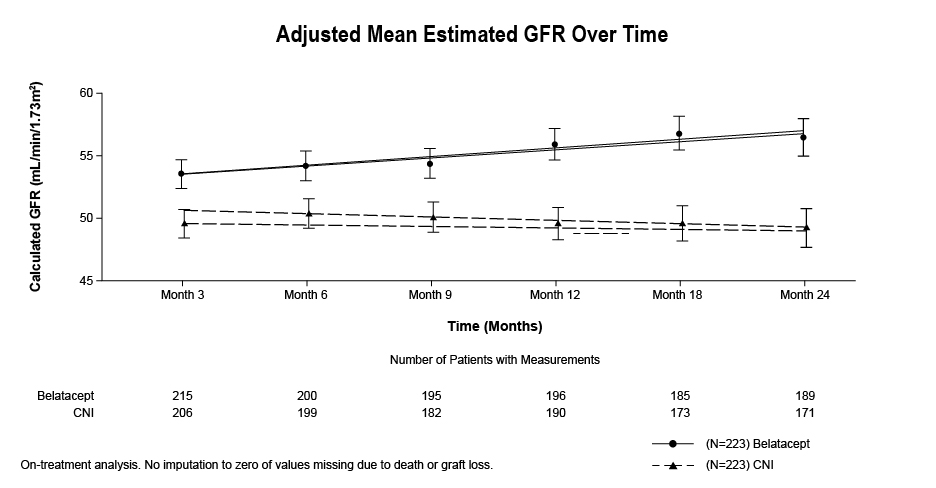
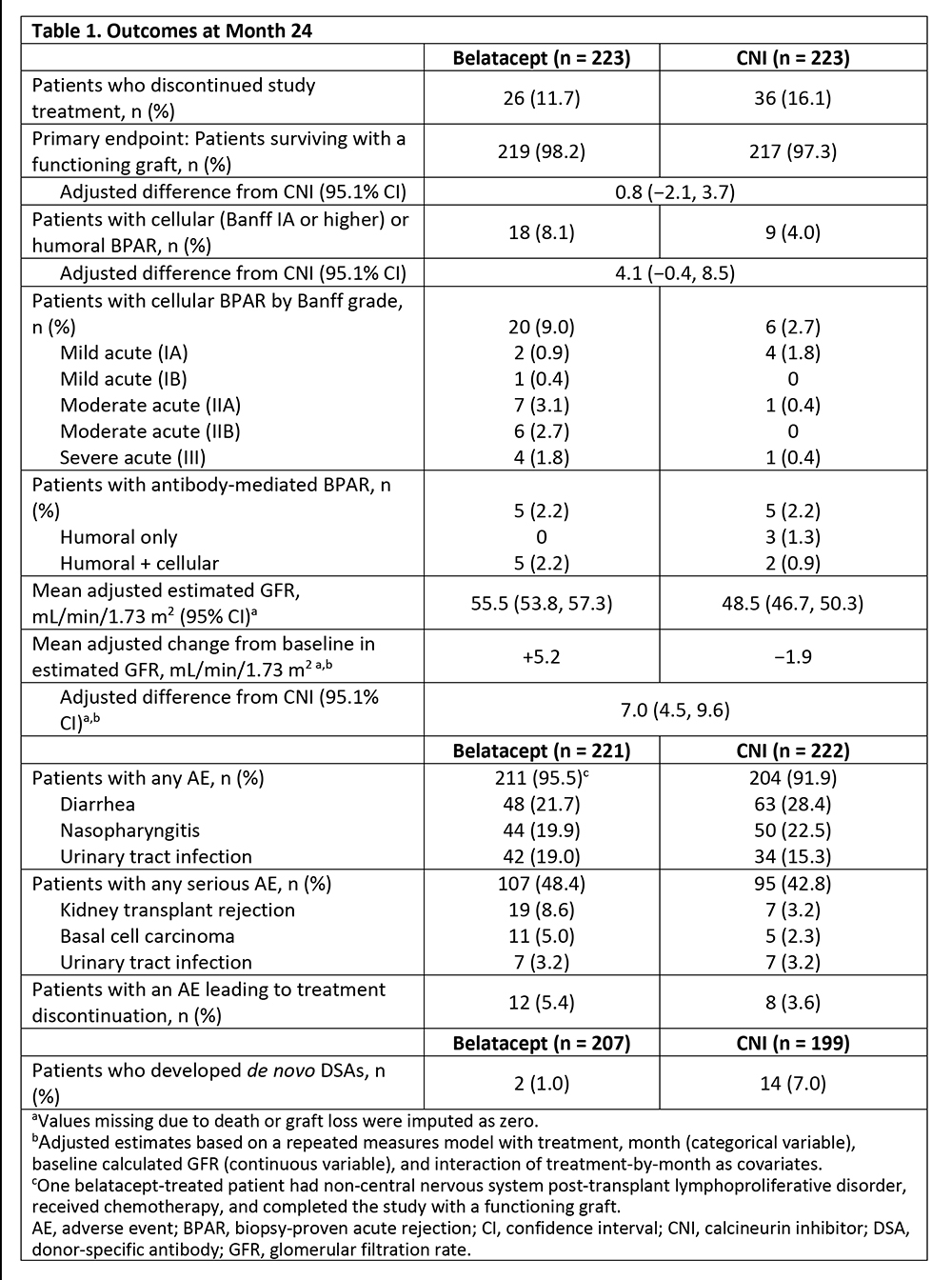
*Results: The proportion of patients surviving with a functional graft to Month 24 was similar for BELA (98.2%) and CNI (97.3%) (Table). The rate of biopsy-proven acute rejection (BPAR) was higher for BELA (8.1%) than CNI (4.0%). At Month 24, estimated GFR was higher for BELA than CNI (55.5 vs 48.3 mL/min/1.73 m2, Figure). No hepatotoxicity, central nervous system infection, serious infusion-related adverse event (AE), or clinically significant blood pressure change was reported. No new or unexpected AE was seen with either regimen.
*Conclusions: Switching stable renal transplant recipients from CNI-based to BELA-based immunosuppression was associated with a similar rate of death or graft loss, improved renal function, and reduced de novo donor-specific antibody formation, albeit with a numerically higher rate of BPAR.
To cite this abstract in AMA style:
Budde K, Prashar R, Haller H, Rial M, Kamar N, Agarwal A, Fijter Jde, Rostaing L, Berger S, Djamali A, Leca N, Allamassey L, Gao S, Polinsky M, Vincenti F. Prospective, Randomized Study of Conversion from Calcineurin Inhibitor (CNI)- to Belatacept (BELA)-Based Maintenance Immunosuppression in Renal Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/prospective-randomized-study-of-conversion-from-calcineurin-inhibitor-cni-to-belatacept-bela-based-maintenance-immunosuppression-in-renal-transplant-recipients/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress