Prospective Evaluation of Donor-Derived Cell-Free DNA (dd-cfDNA) in Monitoring Response to Anti-Rejection Treatment in Kidney Transplant Recipients with Indication Biopsy
1Department of Nephrology, University Hospital Heidelberg, Heidelberg, Germany, 2Transplantation Immunology, Institute of Immunology, University Hospital Heidelberg, Heidelberg, Germany, 3Transplant Immunology Research Center of Excellence, Koç University Hospital, Istanbul, Turkey
Meeting: 2022 American Transplant Congress
Abstract number: 1539
Keywords: Biopsy, Kidney transplantation, Outcome, Rejection
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Donor-derived cell-free DNA (dd-cfDNA) is a marker of allograft injury in kidney transplant recipients (KTR). Little is known about the possible use of dd-cfDNA in evaluating response to anti-rejection treatment in KTR showing histopathological signs of rejection.
*Methods: We are currently prospectively evaluating the diagnostic benefit of dd-cfDNA in 100 KTR undergoing indication biopsy. dd-cfDNA is quantified in the AlloSeq cfDNA assay (CareDx) at time of biopsy to estimate the association of dd-cfDNA levels with histopathological reporting. To assess the utility of dd-cfDNA in monitoring response to therapy, dd-cfDNA levels are quantified after initiation of treatment on days 7, 30, and 90 following biopsy.
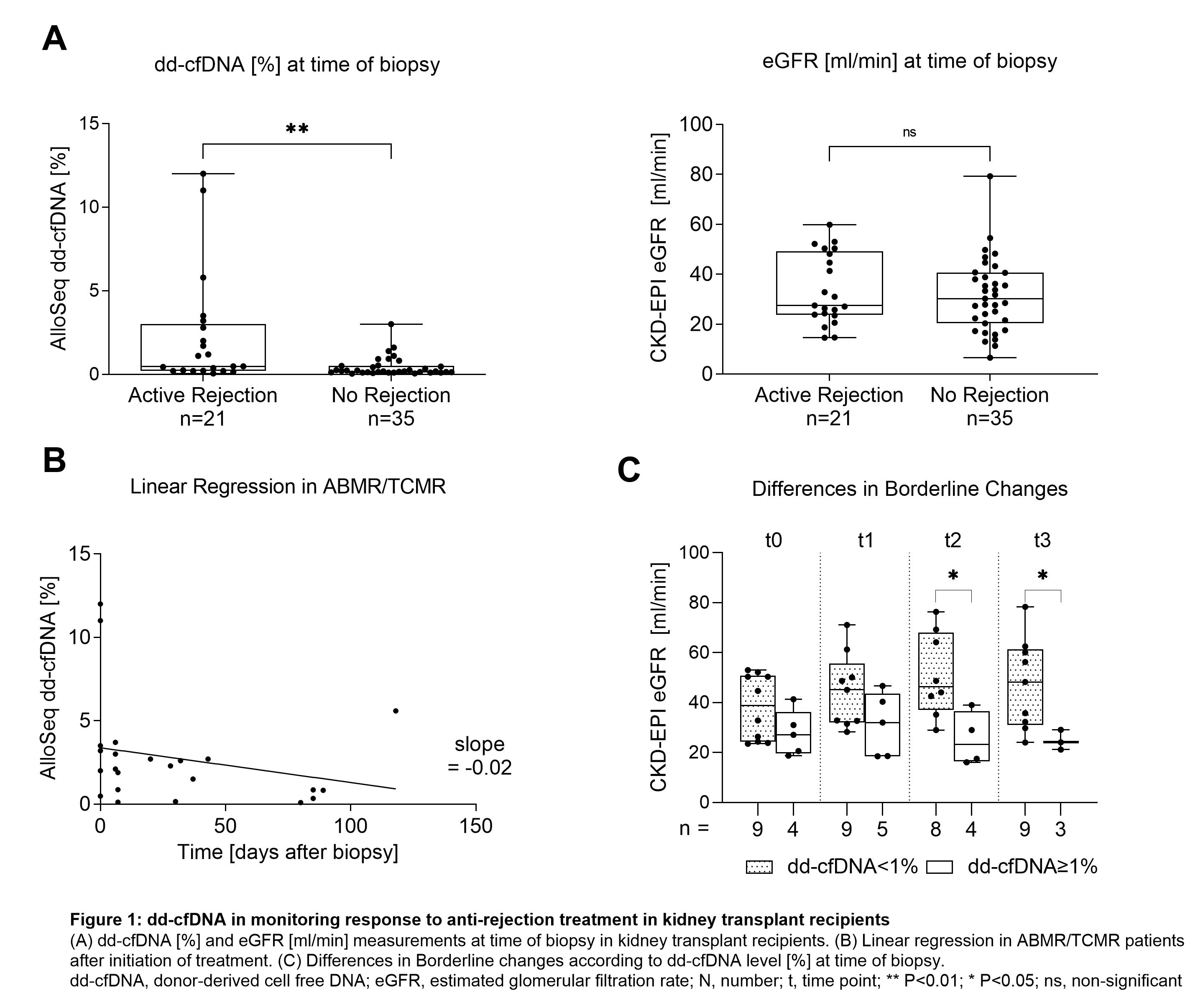
*Results: Since December 2020, 56 KTR have been enrolled and 21/56 (38%) biopsies were graded as different types of rejection. Patients showing signs of active rejection had significantly higher levels of dd-cfDNA at time of biopsy than patients without any signs for rejection, whereas estimated glomerular filtration rate (eGFR) did not differ significantly between the two groups (P<0.01 and P=0.55, respectively, Figure 1A). In patients with antibody-mediated rejection (ABMR) or T-cell mediated rejection (TCMR), median (IQR) dd-cfDNA levels were highest with 3.4% (1.6-11.3) compared to the 0.4% (0.2-1.2) measured in patients with borderline changes and 0.2% (0.1-0.5) in patients with no signs of rejection. When considering only patients with ABMR or TCMR, we observe decreasing levels of dd-cfDNA following initiation of therapy (pooled slope -0.02; Figure 1B). In patients with borderline changes and low levels of dd-cfDNA (<1%) at time of biopsy we see an increase in eGFR after initiation of corticosteroid pulse therapy, whereas patients with high levels of dd-cfDNA (≥1%) show subsequent eGFR decline (Figure 1C).
*Conclusions: dd-cfDNA significantly discriminates active rejection at time of biopsy in KTR. Decreasing levels of dd-cfDNA may indicate treatment response in patients with ABMR and TCMR. dd-cfDNA may further help to identify borderline changes with favorable outcome from changes where additional therapy and closer monitoring is needed.
To cite this abstract in AMA style:
Benning L, Fink A, Kälble F, Speer C, Nusshag C, Zeier M, Süsal C, Morath C, Tran T. Prospective Evaluation of Donor-Derived Cell-Free DNA (dd-cfDNA) in Monitoring Response to Anti-Rejection Treatment in Kidney Transplant Recipients with Indication Biopsy [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/prospective-evaluation-of-donor-derived-cell-free-dna-dd-cfdna-in-monitoring-response-to-anti-rejection-treatment-in-kidney-transplant-recipients-with-indication-biopsy/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress