Primary Outcome of OPTIMAL: A Prospective Multicenter Trial of Immunosuppression Withdrawal (ISW) in Stable Adult Liver Transplant (LT) Recipients
1ITN, San Francisco, CA, 2Rho, Inc., Durham, NC, 3MGH, Boston, MA
Meeting: 2021 American Transplant Congress
Abstract number: LB 17
Keywords: Immunosuppression, Liver transplantation, Tolerance
Topic: Clinical Science » Liver » Liver: Immunosuppression and Rejection
Session Information
Session Name: Late Breaking: All Organs
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 8, 2021
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-6:05pm
Presentation Time: 6:00pm-6:05pm
Location: Virtual
*Purpose: To identify clinical & mechanistic correlates of successful ISW and operational tolerance (OT) in a selected cohort of adult LT recipients.
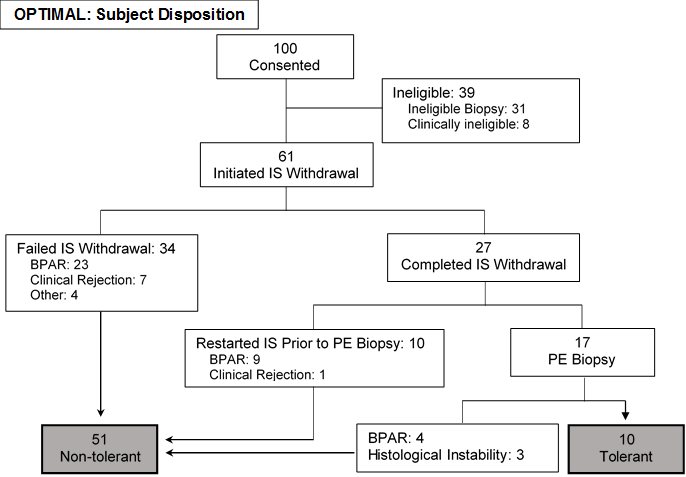
*Methods: The Immune Tolerance Network ITN056ST study (OPTIMAL) enrolled non-autoimmune, non-viral adult LT recipients ≥3 years post-transplant with stable graft function on CNI-based IS. Eligible subjects without significant inflammation/ fibrosis on a screening biopsy underwent phased ISW over 24-35 weeks. The primary endpoint was OT at 52 weeks following complete ISW, defined as no episodes of acute rejection and a liver biopsy showing histologic stability & absence of rejection by Banff criteria.
*Results: Of 92 clinically eligible subjects who underwent screening biopsies, 61 had favorable histology and initiated ISW. Ineligible biopsies had inflammation (n=17) and/or fibrosis (n=9), bile duct damage (n=3), or arteriopathy (n=3). Subjects with favorable biopsies were significantly older at transplant (median age 56 vs. 47 years, p<0.01) and enrollment (median age 64 vs. 54 years, p<0.01), and more likely to have steatohepatitis (50.8% vs. 9.7%, p<0.01). No significant differences were observed in other parameters.
27 subjects completed ISW successfully. Of these, 10 met the primary endpoint (one additional subject did not meet biopsy criteria for OT but remained off IS). 34 subjects failed to complete ISW and 16 subjects restarted IS due to rejection or histologic instability. No significant differences were identified in the baseline clinical characteristics of tolerant and non-tolerant subjects. There were no cases of chronic rejection or graft loss.
| Non-tolerant (n=51) | Tolerant (n=10) | |
| Age (yrs) | 63 (56-68) | 65 (55-69) |
| Time since transplant (yrs) | 7 (5-11) | 9 (4-13) |
| Deceased donor, n (%) | 36 (70.6) | 8 (80) |
| Male, n (%) | 37 (72.5) | 6 (60) |
| White race, n (%) | 43 (84.3) | 7 (70) |
| NASH as cause of liver disease | 6 (11.8) | 2 (20) |
| ALT (IU/L) | 22 (17-29) | 20 (17-25) |
*Conclusions: One in 3 clinically stable, long term adult LT recipients harbors subclinical allograft inflammation or fibrosis. A minority (16.4%; 95% CI: 8.2%-28.1%) of histologically and clinically stable LT recipients can successfully discontinue and remain off IS. Baseline clinical features do not appear to predict ISW outcome. Analyses are underway to identify histologic & mechanistic predictors of OT which could inform strategies to increase the success of ISW in future ITN studies.
To cite this abstract in AMA style:
Chandran S, Mason K, Sun LF, Tanimine N, DesMarais M, Burrell B, Markmann JF. Primary Outcome of OPTIMAL: A Prospective Multicenter Trial of Immunosuppression Withdrawal (ISW) in Stable Adult Liver Transplant (LT) Recipients [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/primary-outcome-of-optimal-a-prospective-multicenter-trial-of-immunosuppression-withdrawal-isw-in-stable-adult-liver-transplant-lt-recipients/. Accessed March 2, 2026.« Back to 2021 American Transplant Congress