Preemptive Therapy for Cytomegalovirus (CMV) Disease Prevention in High-Risk CMV Donor Seropositive Recipient Seronegative (D+/R-) Liver Transplant Recipients in a Real-World Setting
K. M. Doss1, C. Kling1, M. R. Heldman1, N. Singh2, M. Wagener3, C. E. Fisher1, A. Limaye1
1University of Washington, Seattle, WA, 2University of Pittsburgh, PIttsburgh, PA, 3University of Pittsburgh, Pittsburgh, PA
Meeting: 2022 American Transplant Congress
Abstract number: 979
Keywords: Cytomeglovirus, High-risk, Prophylaxis, Viral therapy
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
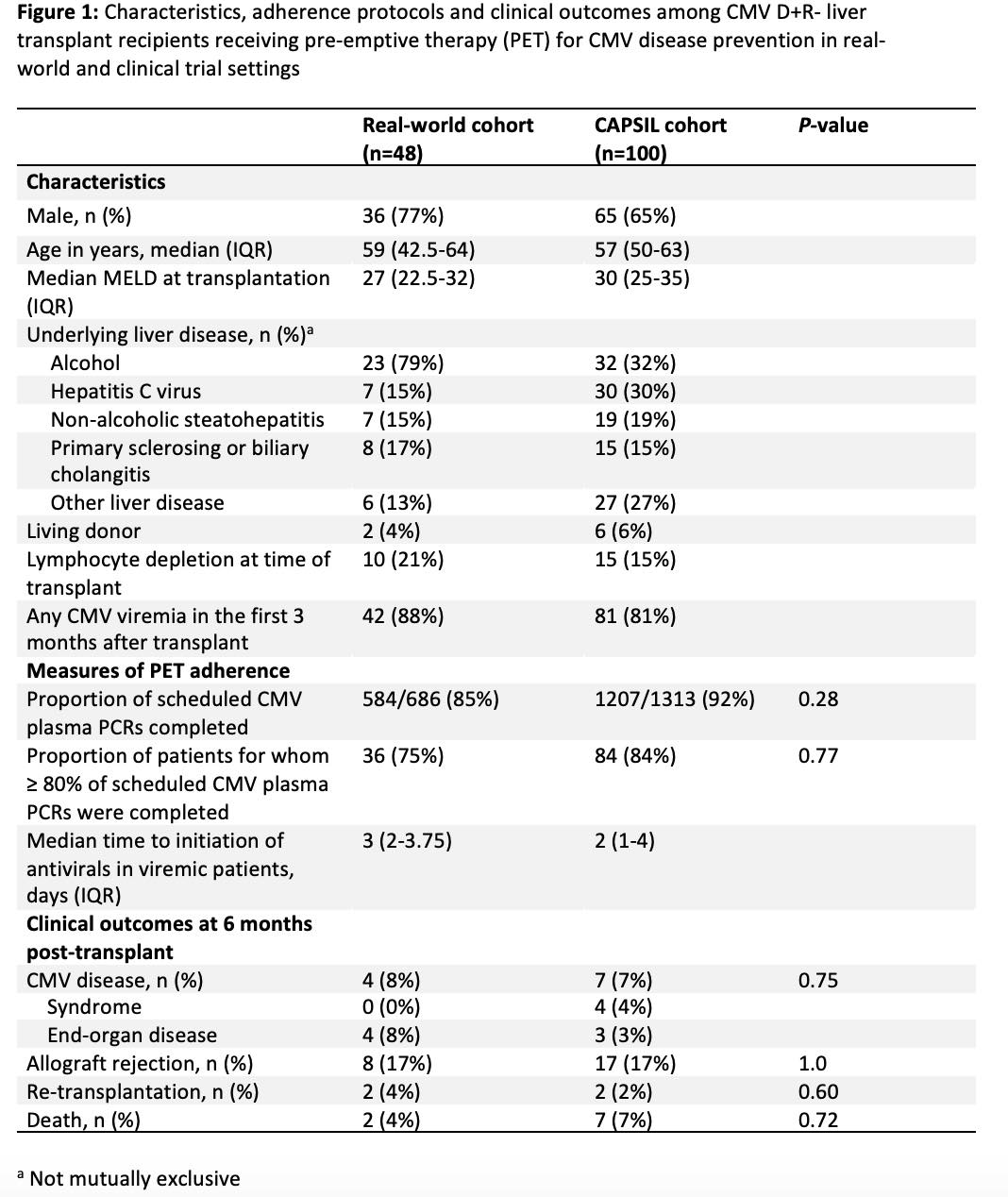
*Purpose: Preemptive therapy (PET) has demonstrated superiority to antiviral prophylaxis for prevention of cytomegalovirus (CMV) disease in CMV donor seropositive/recipient seronegative (D+R-) liver transplant recipients (LTxRs) in clinical trials. However, logistical challenges may preclude PET implementation in clinical practice and the feasibility of PET has not been assessed in real-world settings.
*Methods: We retrospectively assessed a cohort of consecutive adult CMV D+R- LTxRs receiving PET for CMV prevention between 4/4/2019 and 4/18/2021 at a single center (real-world cohort, N=48) and compared measures of adherence to PET protocols and clinical outcomes to a cohort of CMV D+R- LTxRs randomized to PET as part of a clinical trial (CAPSIL, N=100). PET consisted of weekly CMV plasma PCR for 3 months post-transplant with initiation of valganciclovir 900mg twice daily at any detectable level of viremia. Adherence to PET was measured by proportion of required CMV PCRs actually performed, proportion of patients with >80% of scheduled PCRs completed, and time to initiation of antivirals in viremic patients. Clinical outcomes included incidence of CMV disease, allograft rejection, re-transplant and mortality within 6 months of transplant. Chi-square and Fisher’s exact tests were used to compare proportions between groups.
*Results: Incidence of viremia was similar in the real-world and CAPSIL cohorts (88% vs 81%, respectively). There was no significant difference in the proportion of successfully completed CMV plasma PCRs between the real-world (584/686, 85%) and CAPSIL (1207/1313, 92%) cohorts (p=0.28). Antivirals were initiated at a median of 3 days (IQR 2-3.75) in the real-world group versus 2 days (IQR 1-4) in the CAPSIL cohort. CMV syndrome or end-organ disease occurred in 8% of real-world cohort and 7% of LTxRs in the CAPSIL group (p=0.75). There was no significant difference in the incidence of allograft rejection, re-transplantation, or death between groups (Figure 1).
*Conclusions: Adherence to viral load monitoring and timely initiation of antivirals is feasible in LTxRs receiving PET for CMV disease prevention in a real-world setting. Further research is needed to assess the generalizability of these findings in broader clinical contexts.
To cite this abstract in AMA style:
Doss KM, Kling C, Heldman MR, Singh N, Wagener M, Fisher CE, Limaye A. Preemptive Therapy for Cytomegalovirus (CMV) Disease Prevention in High-Risk CMV Donor Seropositive Recipient Seronegative (D+/R-) Liver Transplant Recipients in a Real-World Setting [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/preemptive-therapy-for-cytomegalovirus-cmv-disease-prevention-in-high-risk-cmv-donor-seropositive-recipient-seronegative-d-r-liver-transplant-recipients-in-a-real-world-setting/. Accessed February 23, 2026.« Back to 2022 American Transplant Congress