Pre-Transplant Desensitization Utilizing Intravenous Immune-Globulin and Tocilizumab: A Single Center Experience
Virginia Commonwealth University Health System, Richmond, VA
Meeting: 2022 American Transplant Congress
Abstract number: 403
Keywords: Circulatory Death, IVIG, Kidney transplantation, Sensitization
Topic: Clinical Science » Kidney » 36 - Kidney Immunosuppression: Desensitization
Session Information
Session Name: Kidney Immunosuppression: Desensitization & Acute Antibody Mediated Rejection
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:00pm-4:10pm
Presentation Time: 4:00pm-4:10pm
Location: Hynes Ballroom A
*Purpose: Highly sensitized end stage kidney disease patients face a huge barrier to transplant due to lack of effective pre-transplant desensitization therapies. Various strategies have been studied with variable favorable outcomes. We previously published our experience with intravenous immunoglobulin (IVIg) and rituximab, where we could not show any significant reduction in sensitization or the transplant wait time. A previous single-center study showed a benefit of interleukin 6 receptor antagonist; tocilizumab (TZB) for pre-transplant wait list desensitization. Here we present our initial experience with a modified outpatient protocol of desensitization that included (IVIg) and TZB.
*Methods: Highly sensitized patients (calculated panel reactive antibody; cPRA>80%), who had previously failed desensitization with IVIg+/-rituximab, had no available donors and had at least 1 year of wait time were eligible for the protocol. The desensitization protocol included monthly doses of IVIg (2 g/kg) and TZB 8mg/kg for 6 months. PRA and the mean total antibody levels represented by the relative intensity scale (RIS) originally used by Vo et.al (Transplantation 2015), were obtained at baseline, 3 months, 6 months and 12 months intervals.
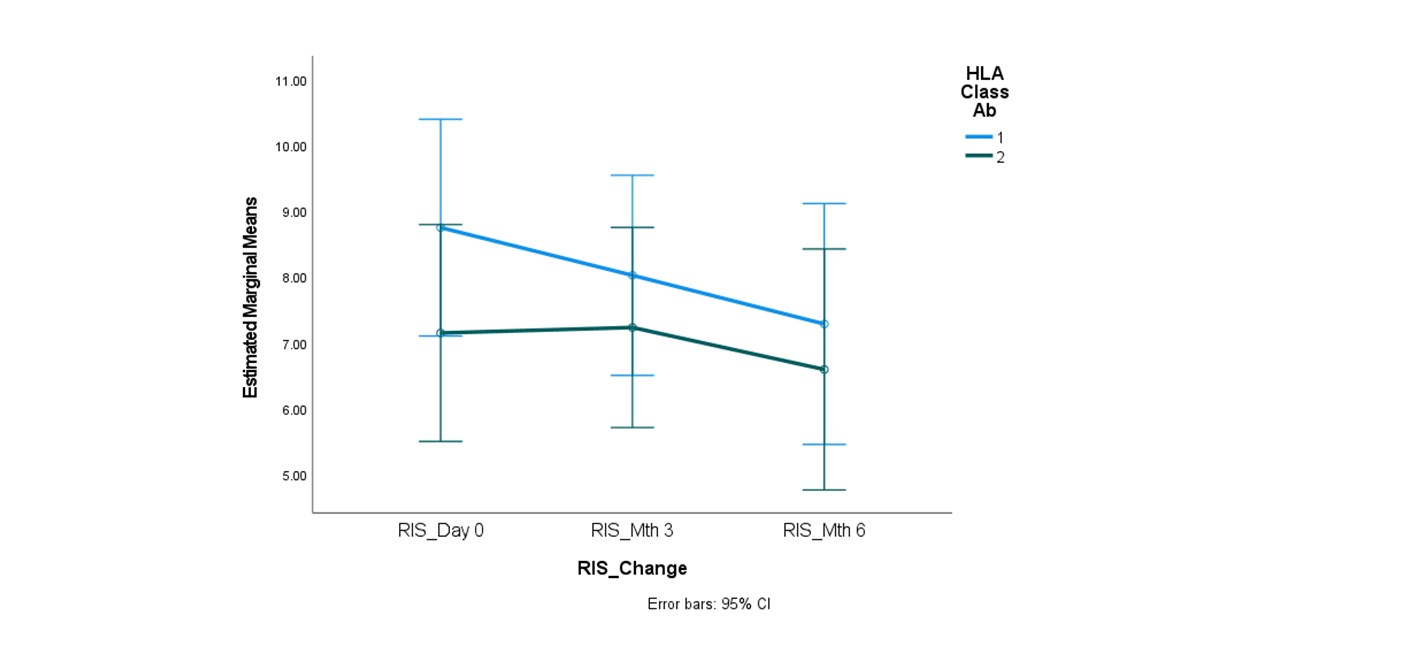
*Results: 15 patients with a median class I PRA of 92% (range=24-100%) and class II PRA of 93% (range=0-100%) underwent desensitization with IVIg and TZB. 87% (13/15) patients were African American, and 67% (10/15) patients had a prior kidney transplant. There was a non-statistically significant trend towards decline in Class I PRA (median from 92% to 83%) and class II PRA (median from 93% to 83%) at the 6 month mark. There was a statistically significant decline in the RIS of class I antibodies (from 8.74 + 2.23 to 7.28 + 3.31; p=0.05) although no significant decline was seen in the RIS for class II antibodies (7.14 + 3.79 to 6.59 + 3.60; p=0.43; Figure 1). There was no significant decline or rebound in PRA or the RIS for both class I and class II beyond 6 months. 4/15 (27%) patients received a kidney transplant at a median of 9 months (range 6-15 months) after starting therapy.
*Conclusions: This small single center real-world study suggests that combination IVIg and TZB may have some efficacy in desensitization of wait listed candidates that have previously failed first line IVIg +/- rituximab. Further follow-up and an increased sample size is needed to clarify whether this protocol increases access to transplant in the current allocation era or whether extending the protocol to beyond 6 months may provide additional efficacy.
To cite this abstract in AMA style:
Moinuddin I, Miles M, Sterling S, Brown A, Kimball P, Gupta G. Pre-Transplant Desensitization Utilizing Intravenous Immune-Globulin and Tocilizumab: A Single Center Experience [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/pre-transplant-desensitization-utilizing-intravenous-immune-globulin-and-tocilizumab-a-single-center-experience/. Accessed February 21, 2026.« Back to 2022 American Transplant Congress