Personalized Tacrolimus (TAC) Dosing Using Genetic Variants in Caucasian Kidney Transplant (Tx) Recipients.
1Dept of Experimental and Clinical Pharmacology, Col of Pharmacy, Univ of Minnesota(UMN), Mpls, MN
2Chronic Disease Research Group, MPLS Medical Research Foundation, Mpls, MN
3Div of Biostatistics, School of Public Health, UMN, Mpls, MN
4Dept of Med Chem, Col of Pharmacy, UMN, Mpls, MN
5Dept of Nephrology, Univ of Alabama, Birmingham, AL
6Div of Transplantation, Dept of Surgery, School of Medicine, UMN, Mpls, MN
7Dept of Nephrology, Hennepin County Medical Center, School of Medicine, UMN, Mpls, MN
Meeting: 2017 American Transplant Congress
Abstract number: D77
Keywords: Genomics, Immunosuppression, Kidney transplantation, Pharmacokinetics
Session Information
Session Name: Poster Session D: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Tuesday, May 2, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
TAC is an immune suppressant with a narrow therapeutic index and high pharmacokinetic (PK) variability leading to uncertainty in blood concentrations. We previously developed an African-American (AA) specific TAC dosing equation using clinical covariates and genetic variants. Here, we present a dosing equation specific for European American (EA) ancestry.
Methods: Kidney tx recipients from 2005-2011 were prospectively followed at 7 study sites. We created a population PK model from 1354 EA individuals with 21,909 TAC doses and troughs in the first 6 months post-tx using NONMEM 7.3. Stepwise regression tested demographic, lab variables, concomitant medications, and single nucleotide polymorphisms (SNPs) for their effect on TAC clearance (CL). Nested random effects accounted for inter-subject and inter-study site variability. The top SNPs were identified through a genome-wide association study.
Results: Time post-tx, concomitant corticosteroid use, rs776746 (CYP3A5*3) and rs4646437 (CYP3A4 G>A) increased TAC CL whereas older age, simultaneous pancreas–kidney (SPK) tx, concomitant anti-CMV drug use, and rs35599367 (CYP3A4*22) decreased TAC CL. The model is: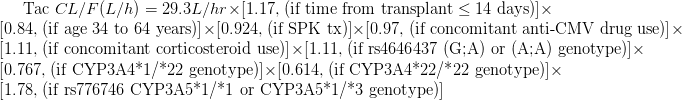 Conclusion: We built a TAC CL model using clinical and genotype data from which dose can be estimated for any desired trough target allowing for personalized dosing. Variants that effect drug CL differ between AA and EA individuals. This model will be validated using a new genomics cohort.
Conclusion: We built a TAC CL model using clinical and genotype data from which dose can be estimated for any desired trough target allowing for personalized dosing. Variants that effect drug CL differ between AA and EA individuals. This model will be validated using a new genomics cohort.
Work was supported by NIAID Grants 5U19-AI070119 and 5U01-AI058013.
CITATION INFORMATION: Margraf D, Brundage R, Schladt D, Guan W, Wu B, Remmel R, Dorr C, Berglund D, Mannon R, Matas A, Oetting W, Israni A, Jacobson P, For the DeKAF Genomics Investigators Personalized Tacrolimus (TAC) Dosing Using Genetic Variants in Caucasian Kidney Transplant (Tx) Recipients. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Margraf D, Brundage R, Schladt D, Guan W, Wu B, Remmel R, Dorr C, Berglund D, Mannon R, Matas A, Oetting W, Israni A, Jacobson P. Personalized Tacrolimus (TAC) Dosing Using Genetic Variants in Caucasian Kidney Transplant (Tx) Recipients. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/personalized-tacrolimus-tac-dosing-using-genetic-variants-in-caucasian-kidney-transplant-tx-recipients/. Accessed July 1, 2025.« Back to 2017 American Transplant Congress
