Peri-Operative Basiliximab is Not Associated with Post-Transplant Hepatocellular Carcinoma Recurrence in Liver Transplant Recipients
1Pharmacy, NewYork-Presbyterian Hospital, New York, NY, 2Surgery, Columbia University Irving Medical Center, New York, NY, 3Pharmacy, Hospital of the University of Pennsylvania, Philadelphia, PA, 4Medicine, Weill Cornell Medicine, New York, NY, 5Surgery, Weill Cornell Medicine, New York, NY
Meeting: 2019 American Transplant Congress
Abstract number: D115
Keywords: Hepatocellular carcinoma, Interleukin-2 receptor, Liver transplantation, Recurrence
Session Information
Session Name: Poster Session D: Liver: Hepatocellular Carcinoma and Other Malignancies
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: The optimal immunosuppression for liver transplant recipients (LTR) with hepatocellular carcinoma (HCC) is unknown. Basiliximab, an interleukin-2 receptor antagonist (IL-2RA), confers immunosuppression for LTR that require a delay in the initiation of calcineurin inhibitors (CNI). Basiliximab has been previously associated with early HCC recurrence, possibly due to inhibitory effects on regulatory T-cells involved in cancer surveillance. The objective of this study was to determine if peri-operative IL-2RA is associated with HCC recurrence post-transplant.
*Methods: All adult LTR with HCC between 2007 and 2014, at a single institution, were assessed. LTR who died within 90 days of transplant were excluded. Patients were stratified by receipt of IL-2RA for delayed CNI introduction. The primary outcome was recurrence-free survival (RFS) at 1 year between the two groups (no IL-2RA vs. IL-2RA). Secondary outcomes included RFS at 3 years and any recurrence.
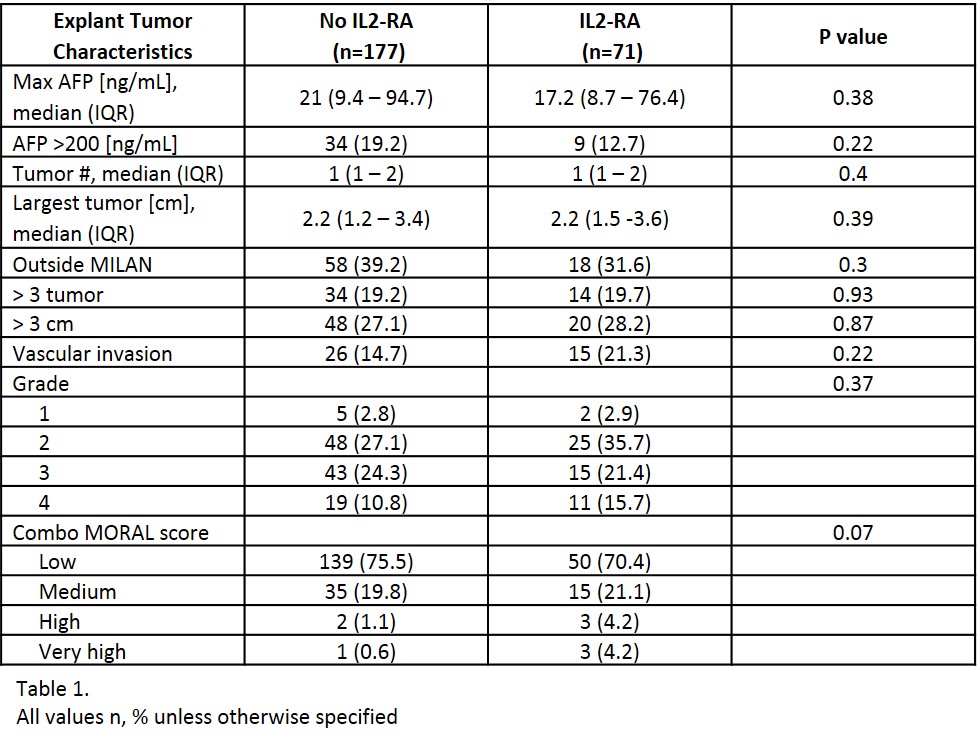
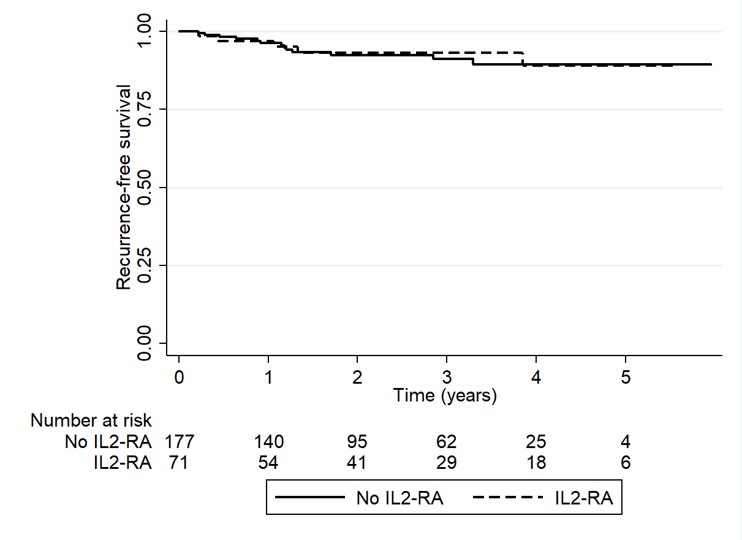
*Results: A total of 248 LTR with pre-transplant HCC were included; 71 patients received peri-operative IL-2RA therapy. There were no differences in baseline characteristics. The median age at transplant was 60 years, 81% were male, and 53% had cirrhosis secondary to HCV. Pre-operative tumor characteristics (AFP, tumor size, tumor number and pre-MORAL score) as well as tumor characteristics on explant (tumor number, largest tumor size, vascular invasion, tumor grade, tumors outside MILAN criteria and combo-MORAL score) were similar between groups [Table 1]. RFS at 1 year and 3 years for no-IL2RA vs. IL2RA was 96.3% vs. 96.9% (p=0.47) and 91.1% vs. 92.9% (p=0.42), respectively. The incidence of HCC recurrence at anytime was 7.3% (n=13) in the no IL2-RA and 7% (n=5) in the IL2-RA group, p=0.93 [Figure 1] with a median follow-up time of 2.3 (1.1-3.7) years.
*Conclusions: There was no association between receipt of IL-2RA and early HCC recurrence.
To cite this abstract in AMA style:
Salerno D, Lange N, Simoneau E, Sammons C, Bley D, Jr RSBrown, Emond J, Halazun K. Peri-Operative Basiliximab is Not Associated with Post-Transplant Hepatocellular Carcinoma Recurrence in Liver Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/peri-operative-basiliximab-is-not-associated-with-post-transplant-hepatocellular-carcinoma-recurrence-in-liver-transplant-recipients/. Accessed March 4, 2026.« Back to 2019 American Transplant Congress