Patient Reported Medication Nonadherence and Tolerability: Results of a Prospective Observational Study
D. Taber, N. Patel, K. Foster, F. Bartlett, C. Perez, H. Meadows, N. Pilch, A. Posadas, K. Soliman, V. Rao, M. Casey, V. Rohan, J. McGillicuddy, S. Nadig, D. Dubay
MUSC, Charleston, SC
Meeting: 2020 American Transplant Congress
Abstract number: 285
Keywords: Adverse effects, Kidney transplantation, Liver
Session Information
Session Name: Psychosocial and Treatment Adherence
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 3:15pm-3:27pm
Presentation Time: 3:15pm-3:27pm
Location: Virtual
*Purpose: Medication nonadherence (MNA) is a major contributor to late allograft loss in transplantation (txp). Immunosuppression therapy is highly effective at preventing rejection yet presents a substantial toxicity burden on txp recipients. There is limited data assessing the correlation between MNA and med tolerability in txp.
*Methods: This was a single center prospective observational longitudinal cohort study including adult txp recipients. After consent, txp patients were administered a validated MNA assessment (Morisky Med Adherence Survey, MMAS) and a validated medication tolerability assessment (Med Side Effect Survey, MSES). Those consenting were also emailed the same surveys every 2 months for up to 36 months post-enrollment (repeated longitudinal component). Generalized linear models were used for analyses, accounting for correlation by repeated measures and time.
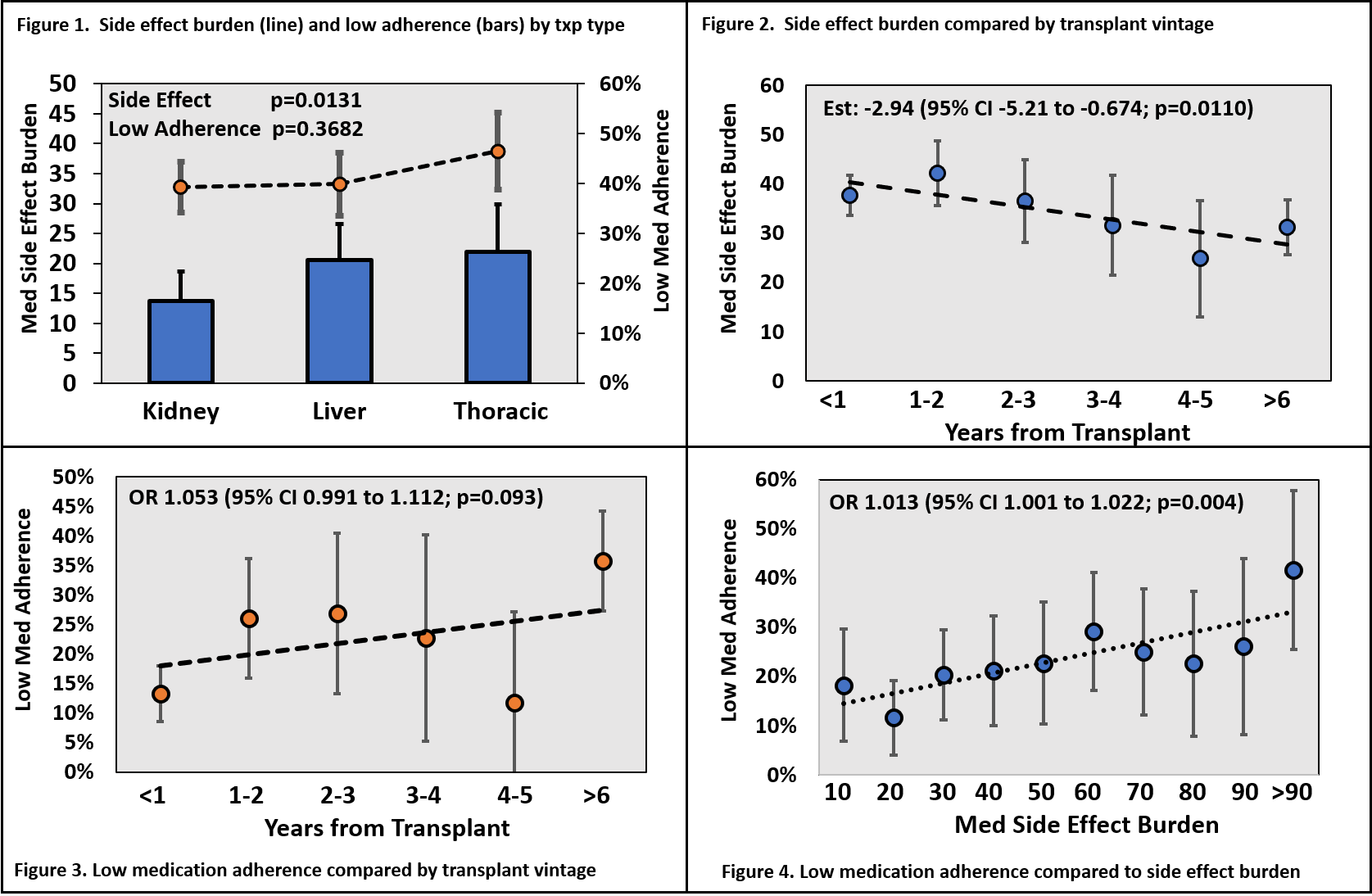
*Results: There were 472 assessments (152 in kidney or panc, 234 in liver and 186 in thoracic) done in 268 txp recipients (106 kidney/panc, 73 liver, 89 thoracic). Mean post-txp time was 4.0±5.4 years and low med adherence was reported in 22.6% of responses. Self-reported medication side effect burden averaged a score of 36±29 (range 0-180, median 29, IQR 12,54) which was higher in liver and thoracic txp as compared to kidney/panc (Fig 1 line; p=0.0131); there were similar rates of self-reported low med adherence across organ type (Fig 1 bars; p=0.3682). Reported med side effect burden improved over time (2.94 reduction in symptom score per txp year; Fig 2; p=0.0110), while low med adherence had an increasing trend over time (5.3% increase per year; Fig 3; p=0.093). Importantly, self-reported medication side effect burden significantly correlated with low medication adherence. For every one-point increase in side effect burden there was a 1.3% corresponding increase in odds of reporting low med adherence (Fig 4; OR 1.013, 95% CI 1.001-1.022; p=0.004). Those with high medication side effect burden (above the mean) had more than twice the odds of reporting low med adherence (OR 2.35, 95%CI 1.39 to 4.00; p=0.0016, model C-statistic 0.656).
*Conclusions: Low medication adherence is reported in nearly one in four transplant recipients, regardless of transplant type. Self-reported medication side effect burden is significantly correlated with low medication adherence and this offers a potential area of intervention for clinicians to improve non-adherence rates.
To cite this abstract in AMA style:
Taber D, Patel N, Foster K, Bartlett F, Perez C, Meadows H, Pilch N, Posadas A, Soliman K, Rao V, Casey M, Rohan V, McGillicuddy J, Nadig S, Dubay D. Patient Reported Medication Nonadherence and Tolerability: Results of a Prospective Observational Study [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/patient-reported-medication-nonadherence-and-tolerability-results-of-a-prospective-observational-study/. Accessed March 1, 2026.« Back to 2020 American Transplant Congress