Pancreatic Mesenchymal Stromal Cells (MSC) Contain a Subset of Islet Progenitor Cells Capable of Regenerating Islets and Reversing Diabetes
1Institute of Biomedical Studies, Waco, TX, 2Baylor University Medical Ctr, Dallas, TX, 3Transplant Surgery Department, VCU, Richmond, VA
Meeting: 2020 American Transplant Congress
Abstract number: 429
Keywords: Islets, N/A, Pancreas, Stem cells
Session Information
Session Name: Islet/Cell Transplantation
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:03pm-4:15pm
Presentation Time: 4:03pm-4:15pm
Location: Virtual
*Purpose: To identity islet precursor cells residing in pancreatic MSC culture preparations that contribute to islet regeneration and restoration of islet cell function in both in vitro and in vivo.
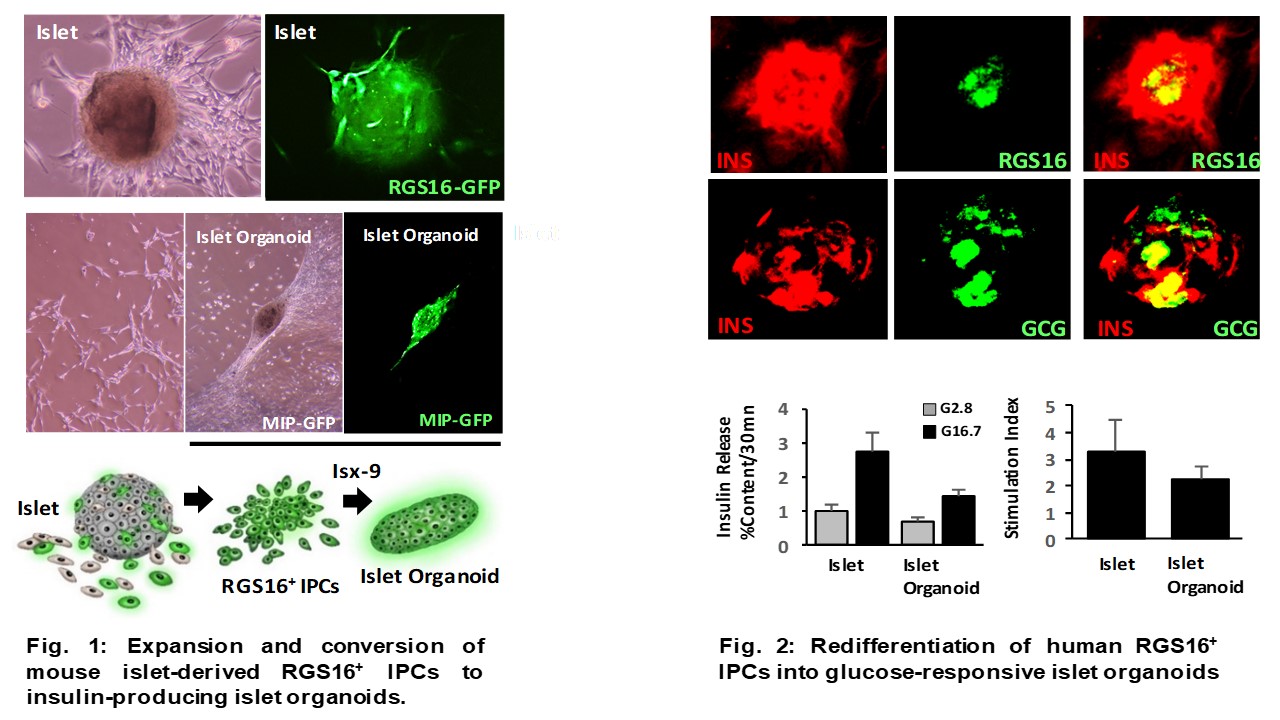
*Methods: Human MSCs were cultured from collagenase digested pancreatic tissue from patients undergoing TPIAT. Human MSCs were co-transplanted with marginal human islet doses to the subcapsular kidney in an STZ-diabetic mouse xenotransplant model. Differentiation of cell subtypes in MSC cultures by isoxazole-9 (Isx-9) was assessed by flow cytometry, qPCR, immunoblot, and immunohistochemistry. Tracing of islet progenitor cell differentiation of islet cell organoids in vitro was achieved by isolation and expansion of MSCs from mouse insulin promoter (MIP) and RGS16-GFP transgenic mice.
*Results: Human MSCs co-transplanted with a marginal dose of human islets (1000 IEQ) reversed diabetes in recipient mice for up to 30 days in contrast to marginal islets alone that remained diabetic. Notably, recipients maintained partial islet cell function after graft removal compared to recipients of islets alone. Sectioning of the native pancreas in these mice showed the presence of human HLA1+ islet-like clusters that expressed human insulin. Analysis of MSCs revealed a subpopulation of RGS16-expressing islet precursor cells (IPCsRGS16+) that selectively differentiated into insulin-producing organoids within 14 days in response to Isx-9 (Fig. 1). This required NFATc2-dependent activation of RFX6 and NeuroD1 to induce transcriptional programing selective for islet cell differentiation. Islet organoids expressed both insulin and glucagon and exhibited glucose-stimulated insulin secretion with stimulation indices comparable to islets (Fig. 2).
*Conclusions: IPCsRGS16+ residing in MSC cultures can migrate to the damaged pancreas to regenerate and restore islet cell function. IPCsRGS16+ can be expanded and selectively activated to induce glucose-responsive islet organoids in vitro by Isx-9. Thus, IPCsRGS16+ may provide a new expandable source of islet tissue for islet cell replacement strategies.
To cite this abstract in AMA style:
Darden CM, Vasu S, Kumano K, Liu Y, Saravanan P, Naziruddin B, Lawrence M. Pancreatic Mesenchymal Stromal Cells (MSC) Contain a Subset of Islet Progenitor Cells Capable of Regenerating Islets and Reversing Diabetes [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/pancreatic-mesenchymal-stromal-cells-msc-contain-a-subset-of-islet-progenitor-cells-capable-of-regenerating-islets-and-reversing-diabetes/. Accessed February 17, 2026.« Back to 2020 American Transplant Congress