Oral versus Intravenous Mycophenolate Mofetil Administration: A Single Center Experience on Incidence of Acute Kidney Injury
1Division of Pulmonary Critical and Sleep Medicine, University of Southern California, Los Angeles, CA, 2Department of Clinical Pharmacy, University of Southern California School of Pharmacy, Los Angeles, CA
Meeting: 2020 American Transplant Congress
Abstract number: B-295
Keywords: Immunosuppression, Lung transplantation, Mycophenolate mofetil, Renal injury
Session Information
Session Name: Poster Session B: Lung: All Topics
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Mycophenolate mofetil (MMF) is one of the main immunosuppressive medications given in lung transplantation (LTx). It is given orally (PO) or intravenously (IV). In heart transplantation patients, similar pharmacokinetics and bioavailability have been reported in either route of administration, but no data exists for LTx. Based on our center’s experience, we suspected a higher incidence of acute kidney injury (AKI) with IV MMF when patients were unable to receive medications orally. This study examines the incidence of AKI in LTx patients who received either oral or IV MMF perioperatively.
*Methods: This study is a single center (Keck Medical Center at University of Southern California), retrospective chart review comparing the incidence of AKI among LTx patients who received oral or IV MMF. Review period was from January 2017 to December 2019. Renal functions immediately pre- and post-transplantation were reviewed. The incidence of AKI was defined by the Kidney Disease Improving Global Outcome (KDIGO) guidelines.
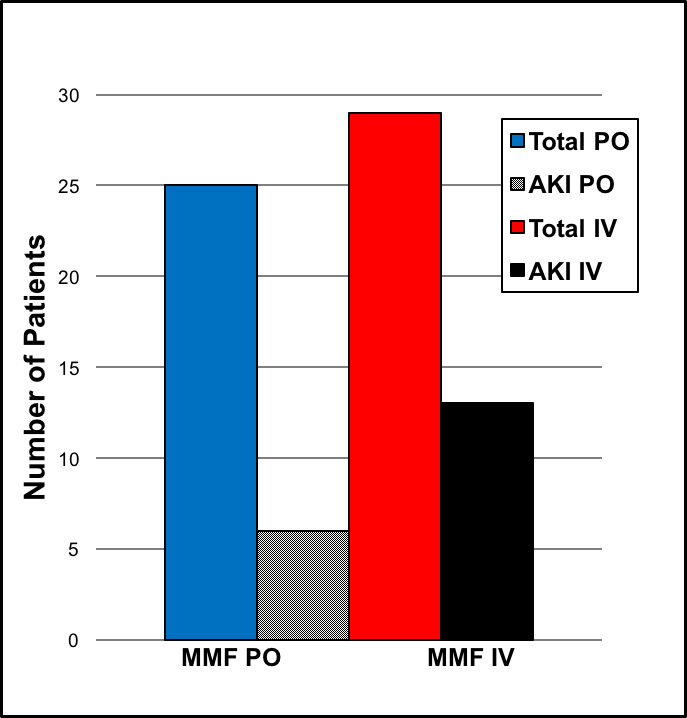
*Results: During the review period, a total of 54 patients underwent LTx. Among them, 25 patients received oral MMF, and 29 patients received IV MMF. The average serum creatinine (SCr) level increased by 0.02mg/dL in the oral group and by 0.04mg/dL in the IV group. AKI was noted in 6 patients in the oral group and 13 patients in the IV group (24% vs. 45%). However, the oral group with AKI had a higher increase in absolute SCr values compared to IV group with AKI (0.25mg/dL vs. 0.18mg/dL).
*Conclusions: There is lack of data and guidelines for optimal route of MMF administration in LTx patients. While there are multiple confounding factors such as duration on cardiopulmonary bypass machine, hemodynamics, degree of blood loss, number of received blood products, and other nephrotoxic medications that affect renal function, only the route of administration was examined in this study. The incidence of AKI was almost twice as much in the IV group compared to the oral group. However, AKI was more severe in the oral group based on absolute values of increased SCr levels. We propose the possibility of different effect of oral vs. IV MMF on renal function perioperatively. The number of patients in this study is small, but the observation and trend reported here should lead to further studies examining the optimal MMF administration route as it may affect the pharmacokinetics and bioavailability of immunosuppression as well as LTx outcome.
To cite this abstract in AMA style:
Chung P, Forrester K, Ganesh S. Oral versus Intravenous Mycophenolate Mofetil Administration: A Single Center Experience on Incidence of Acute Kidney Injury [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/oral-versus-intravenous-mycophenolate-mofetil-administration-a-single-center-experience-on-incidence-of-acute-kidney-injury/. Accessed February 22, 2026.« Back to 2020 American Transplant Congress