Opportunities in Tacrolimus Therapeutic Drug Monitoring
Department of Pharmacy, NewYork-Presbyterian Hospital, NY.
Meeting: 2015 American Transplant Congress
Abstract number: C66
Keywords: Dosage, Kidney transplantation, Pharmacoeconomics, Pharmacokinetics
Session Information
Session Name: Poster Session C: Immunosuppression/Compliance
Session Type: Poster Session
Date: Monday, May 4, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Tacrolimus requires diligent therapeutic drug monitoring (TDM) to maintain appropriate serum concentrations after transplant. To optimize efficacy & minimize toxicity frequent levels are drawn, commonly on a daily basis for inpatients. This analysis identifies areas of improvement from current TDM practices to develop an efficacious & cost-effective tacrolimus dosing & TDM algorithm.
Single-center, retrospective study of 100 adult renal allograft recipients transplanted from Jan–Jun 2013. Information on tacrolimus dosing & serum trough levels were collected from the day of transplant to the day of hospital discharge or post-operative day 14. Monitoring practices were deemed appropriate if the level was obtained 0–30 min prior to a morning dose of tacrolimus once steady state was reached (2.5days of consistent dosing). A cost analysis was determined by comparing the difference of total tacrolimus levels drawn versus the number of levels appropriately obtained.
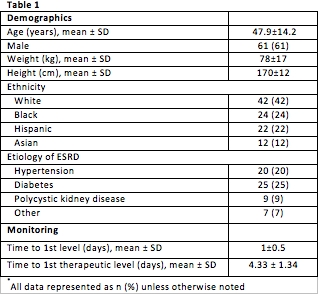
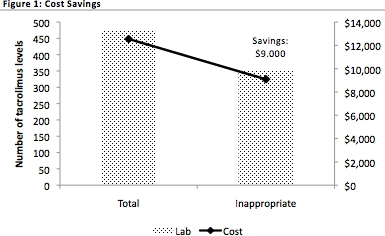
Demographics summarized in Table 1. During the study period 475 tacrolimus levels were drawn. Of these, 352 were deemed inappropriate for the following reasons: tacrolimus level not at steady state & level was drawn (n=333); medication at steady state & therapeutic & level was drawn (n=19). Mean inpatient length of stay was 6±3days. Liberal use of TDM had a significant cost burden, $12,000 on our institution. An algorithm to standardize & encourage appropriate tacrolimus lab draws was developed. If the algorithm was applied to our current cohort, this would lead to $9,000 in cost-savings.
We identified a high incidence of inappropriate TDM (74%) among our renal transplant recipients. Standardized TDM practices are necessary to provide optimal care & to ensure efficacy, safety, & cost-effectiveness. Prospective monitoring of the TDM algorithm for all transplant recipients at our center is ongoing.
To cite this abstract in AMA style:
Jandovitz N, Lee J, Hammad S, McKeen J, Martin S, Tsapepas D. Opportunities in Tacrolimus Therapeutic Drug Monitoring [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/opportunities-in-tacrolimus-therapeutic-drug-monitoring/. Accessed October 23, 2024.« Back to 2015 American Transplant Congress
