Opioid Use Policies and Practices in Liver Transplant and Lung Transplant Candidates: A Comparison of National Surveys
1University of Virginia, Charlottesville, VA, 2University of North Carolina, Chapel Hill, NC, 3University of Pittsburgh, Pittsburgh, PA, 4Medical University of South Carolina, Charleston, SC
Meeting: 2019 American Transplant Congress
Abstract number: D175
Keywords: Liver transplantation, Lung transplantation, Public policy, Waiting lists
Session Information
Session Name: Poster Session D: Non-Organ Specific: Disparities to Outcome and Access to Healthcare
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
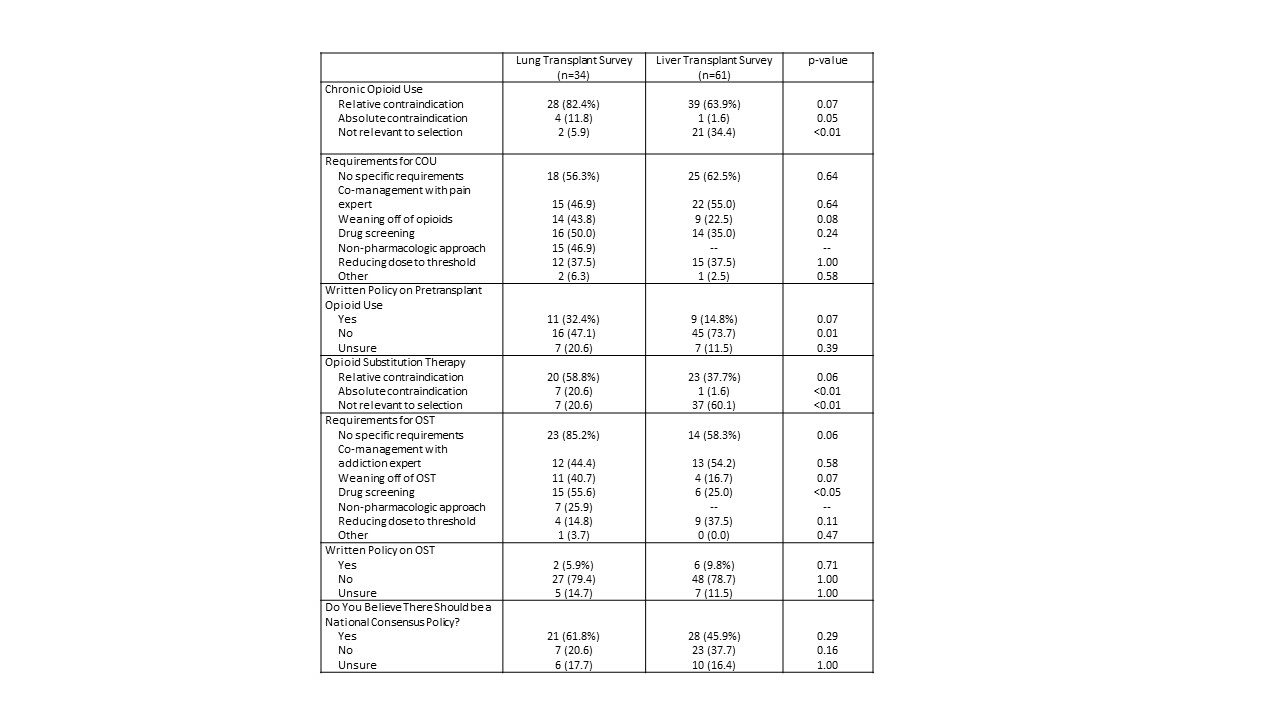
*Purpose: Pre-transplant opioid use has been associated with increased risk of morbidity and mortality. Alternatively, opioid substitution therapy (OST) has been proven to be a successful treatment for opioid addiction. Many centers do not have policies pertaining to this issue and there is no consensus or uniformity of practice in any organ transplant population.This abstract compares two national surveys of policies and practices regarding pre-transplant opioid and OST use: a 2017 survey of liver transplant centers and a similarly structured 2018 survey of lung transplant centers.
*Methods: Surveys were developed and electronically administered to active U.S. liver and lung transplant centers. Descriptive statistics were used.
*Results: The liver transplant survey had a response rate of 53.5% (61 liver centers) and the lung transplant survey had a response rate of 53.1% (34 lung centers). Only one liver transplant center (1.6%) considered opioid use an absolute contraindication, in contrast to four lung transplant centers (11.8%). The majority of liver and lung transplant centers considered opioid use a relative contraindication (63.9% and 82.4%, respectively). One liver center (1.6%) considered OST an absolute contraindication, compared to seven lung centers (20.6%). The majority of lung centers (58.8%) considered OST a relative contraindication, in contrast to 37.7% of liver programs. Only 14.8% of liver programs and 32.4% of lung programs had written policies regarding opioid use, and even fewer centers had a policy regarding OST (9.8% of liver programs, 5.9% of lung programs). A majority of lung survey respondents (61.8%) and 45.9% of liver survey respondents believed that a national consensus policy is needed.
*Conclusions: There is significant variability in policies and practices related to opioid use across liver and lung transplant programs, perhaps due to differing concerns on opioid AE post-transplant. The lack of written policies increases the risk of intracenter variability in the handling of each transplant evaluation. The apparent concern regarding OST use in both populations is concerning given evidence on the risk of recidivism after reduction or elimination of therapy. In addition, there is a strong interest in a national consensus policy regarding opioid use. These findings highlight an opportunity for the transplant community to address this important issue.
To cite this abstract in AMA style:
Bruschwein H, Andersen V, Patel H, Rogal SS, Fleming JN. Opioid Use Policies and Practices in Liver Transplant and Lung Transplant Candidates: A Comparison of National Surveys [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/opioid-use-policies-and-practices-in-liver-transplant-and-lung-transplant-candidates-a-comparison-of-national-surveys/. Accessed March 7, 2026.« Back to 2019 American Transplant Congress