Novel Avenue of Allograft Monitoring: Direct Measurement of Donor-Derived Extracellular Vesicles in Human Plasma
Internal Medicine, Erasmus MC Transplant Institute, Rotterdam, Netherlands
Meeting: 2022 American Transplant Congress
Abstract number: 469
Keywords: Biopsy, FACS analysis, HLA antigens, Kidney
Topic: Basic Science » Basic Clinical Science » 17 - Biomarkers: Clinical Outcomes
Session Information
Session Name: Biomarkers: Clinical Outcomes I
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:30pm-4:40pm
Presentation Time: 4:30pm-4:40pm
Location: Hynes Veterans Auditorium
*Purpose: Extracellular Vesicles (EV) – regarded as “snapshots” of their cell of origin – represent promising liquid biomarkers to monitor allograft function post transplantation. Recently, we developed an imaging flow cytometry (IFCM) based protocol to identify and characterize EV ≤240 nm in molecularly complex samples such as human plasma without prior isolation of EV. Using this protocol, we measure allograft derived EV based on HLA phenotype as a first step to detect allograft specific EV in the circulation of kidney transplant (KTx) recipients.
*Methods: EDTA blood samples from kidney transplant donors (HLA-A2+, n=21) and recipients (HLA-A2-, n=33) were collected before transplantation as well as 3 days, 7 days, 6 months and during ‘for-cause’ biopsies (recipients only) after transplantation. Platelet-poor plasma (PPP) was stained with a donor-specific HLA antibody (HLA-A2) in combination with a common EV marker (tetraspanin CD9) and measured using standardized IFCM.
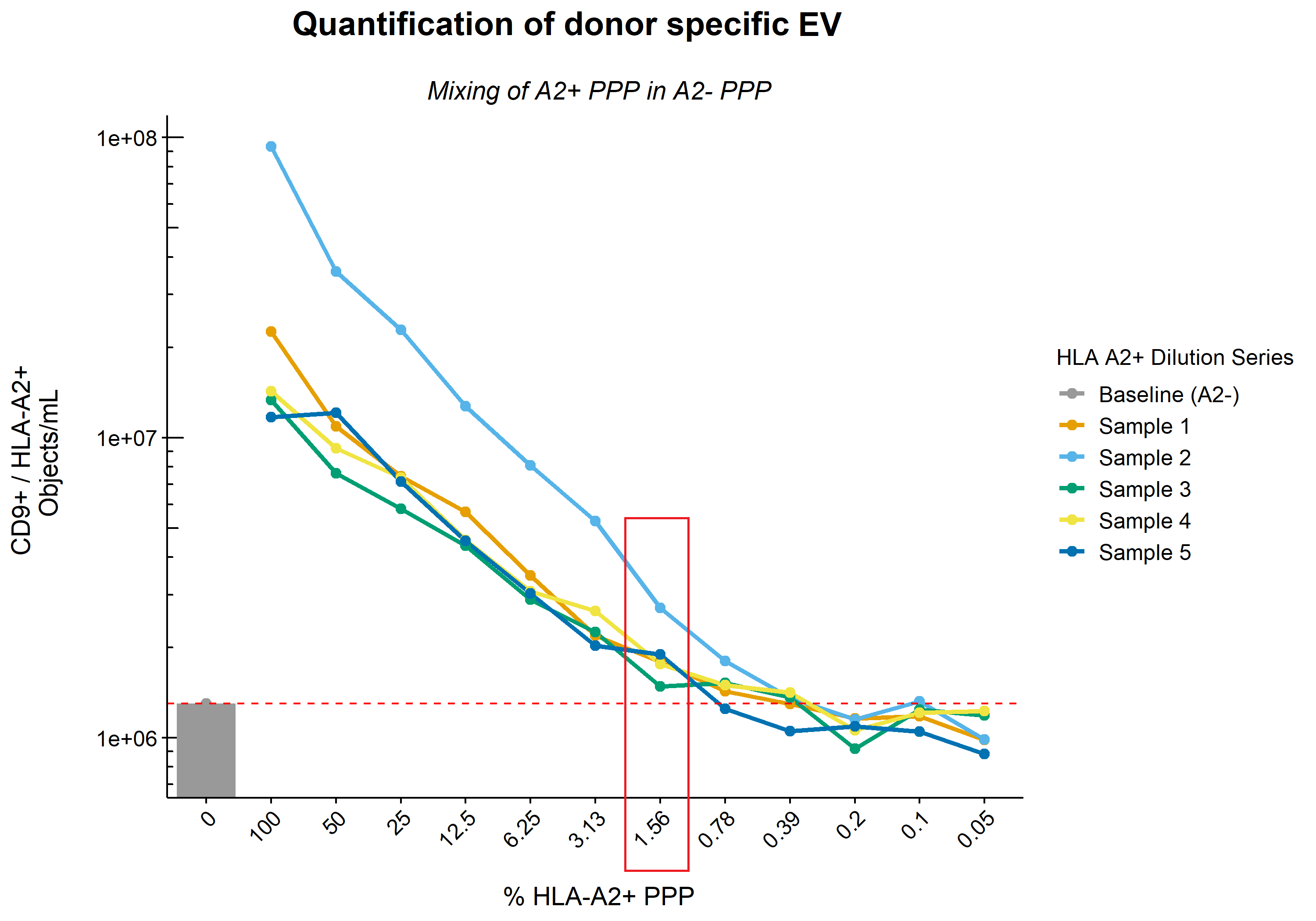
*Results: Quantification and comparison of CD9+/HLA-A2+ double-positive EV showed 1.1E7 ± 8.9E6 vs 3.5E5 ± 2.5E5 objects/mL for donor and recipient (pre-KTx) EV respectively, with recipients A2- EV concentrations representing background level of the machine. CV values for inter- and intra-assay variability were 16% and 11%, respectively. Serial dilution of A2+ PPP in A2- PPP (n=5) showed a linear reduction in the numbers of CD9+/HLA-A2+ EV according to the dilution rate whilst total CD9+ EV levels remained unchanged. The lower limit of detection of IFCM was defined as the dilution at which point CD9+/HLA-A2+ EV dropped below baseline (A2- PPP) and was determined to be ~1%. Measurement of longitudinally collected recipient samples revealed the detection of allograft derived EV as soon as 3 days – but up to at least 6 months – after KTx.
*Conclusions: Here we demonstrate for the first time the detection of allograft derived EV in the circulation of KTx recipients in unprocessed human plasma samples. Identification, quantification and characterization of these EV opens up the possibility to monitor these EV over time after transplantation, and may prove to be a minimally-invasive biomarker.
To cite this abstract in AMA style:
Woud WW, Hesselink DA, Hoogduijn MJ, Baan CC, Boer K. Novel Avenue of Allograft Monitoring: Direct Measurement of Donor-Derived Extracellular Vesicles in Human Plasma [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/novel-avenue-of-allograft-monitoring-direct-measurement-of-donor-derived-extracellular-vesicles-in-human-plasma/. Accessed March 8, 2026.« Back to 2022 American Transplant Congress