mTOR Inhibition with Everolimus in De Novo Living Donor Liver Transplantation – 24-Months Results from the H2307 Pivotal Trial
1H2307 Study Group, Taichung, Taiwan
2Novartis Pharma AG, Basel, Switzerland
3Novartis Pharma KK, Tokyo, Japan.
Meeting: 2018 American Transplant Congress
Abstract number: 61
Keywords: Efficacy, Immunosuppression, Liver transplantation, Safety
Session Information
Session Name: Concurrent Session: Liver: Immunosuppression and Rejection
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Room 6B
Purpose: Everolimus (EVR) with reduced-dose tacrolimus (rTAC) has been shown to be efficacious in preventing graft rejection and improving renal function in deceased-donor liver transplant (LT) recipients. Data on its effectiveness in living-donor (LD) LT is lacking.
Methods: H2307 (NCT01888432) is a 24-month (M), multicenter, open-label trial in adult de novo LDLT recipients who were randomized on Day 30±5 to receive EVR+rTAC or standard-dose TAC (TAC-C). The primary objective was to compare composite efficacy failure (CEF) of treated biopsy-proven acute rejection (tBPAR), graft loss (GL), or death in EVR+rTAC vs TAC-C arms at M12 post-LDLT. Important other objectives were to determine renal function and hepatocellular carcinoma (HCC) recurrence at M12 and M24 follow-up.
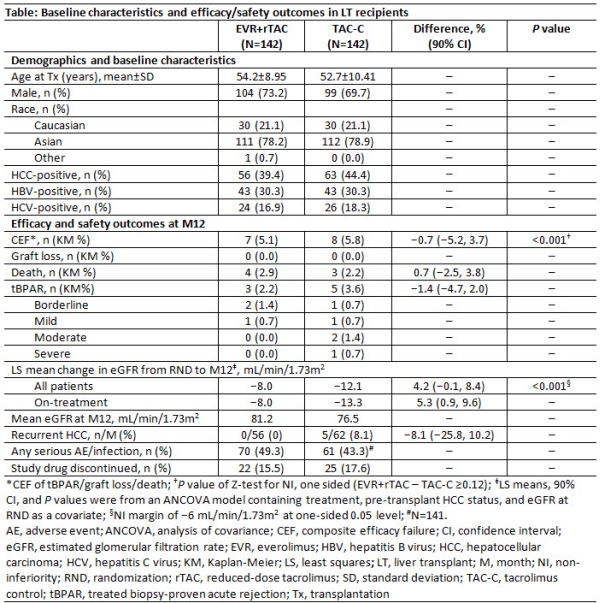
Results: 284 patients were randomized (mean age, 54 years; 72% male; 79% Asian). The MELD score was 14±5; 42% had hepatocellular carcinoma (HCC) and 14% hepatitis B. Baseline characteristics were comparable between the arms (Table). At M12, CEF and patient survival were comparable between the arms without any GL, despite >35% TAC reduction in EVR+rTAC arm. Although tBPAR rates were similar; they were mild in EVR+rTAC arm whereas moderate to severe in TAC-C arm at M12. Non-inferiority of EVR+rTAC to TAC-C for CEF rate was demonstrated at M12. Estimated glomerular filtration rate (eGFR) was numerically and constantly higher throughout the study with EVR+rTAC up to M12. HCC recurrence was only seen in patients in the TAC-C arm (n=5, 8.1%) at M12. Overall serious adverse events/infections were comparable and study drug discontinuation was very low. M24 data will be available in Dec 2017.
Conclusions: Early introduction of EVR by Day 30±5 after LDLT provides similar efficacy despite a >35% TAC reduction. Patients in the EVR+rTAC arm had improved renal function and no HCC recurrence. 24M data which will be shortly available will provide more insights on long-term outcomes on the use of EVR in patients undergoing LDLT.
CITATION INFORMATION: Jeng L., Lee S., Soin A., Uemoto S., Maluf D., Emond J., Meier M., Kochuparampil J., Sips C., Kaneko S., Levy G. mTOR Inhibition with Everolimus in De Novo Living Donor Liver Transplantation – 24-Months Results from the H2307 Pivotal Trial Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Jeng L, Lee S, Soin A, Uemoto S, Maluf D, Emond J, Meier M, Kochuparampil J, Sips C, Kaneko S, Levy G. mTOR Inhibition with Everolimus in De Novo Living Donor Liver Transplantation – 24-Months Results from the H2307 Pivotal Trial [abstract]. https://atcmeetingabstracts.com/abstract/mtor-inhibition-with-everolimus-in-de-novo-living-donor-liver-transplantation-24-months-results-from-the-h2307-pivotal-trial/. Accessed March 4, 2026.« Back to 2018 American Transplant Congress