MHC II-Decoy Car-T Cells to Induce Long-Term Allotransplant Organ Tolerance
1Department of Pharmacology and Experimental Therapeutics, Sidney Kimmel Medical College, Philadelphia, PA, 2Department of Surgery, Division of Transplantation, Sidney Kimmel Medical College, Philadelphia, PA
Meeting: 2020 American Transplant Congress
Abstract number: A-383
Keywords: Histocompatibility antigens, Immune deviation, T cell receptors (TcR)
Session Information
Session Name: Poster Session A: Tolerance / Immune Deviation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
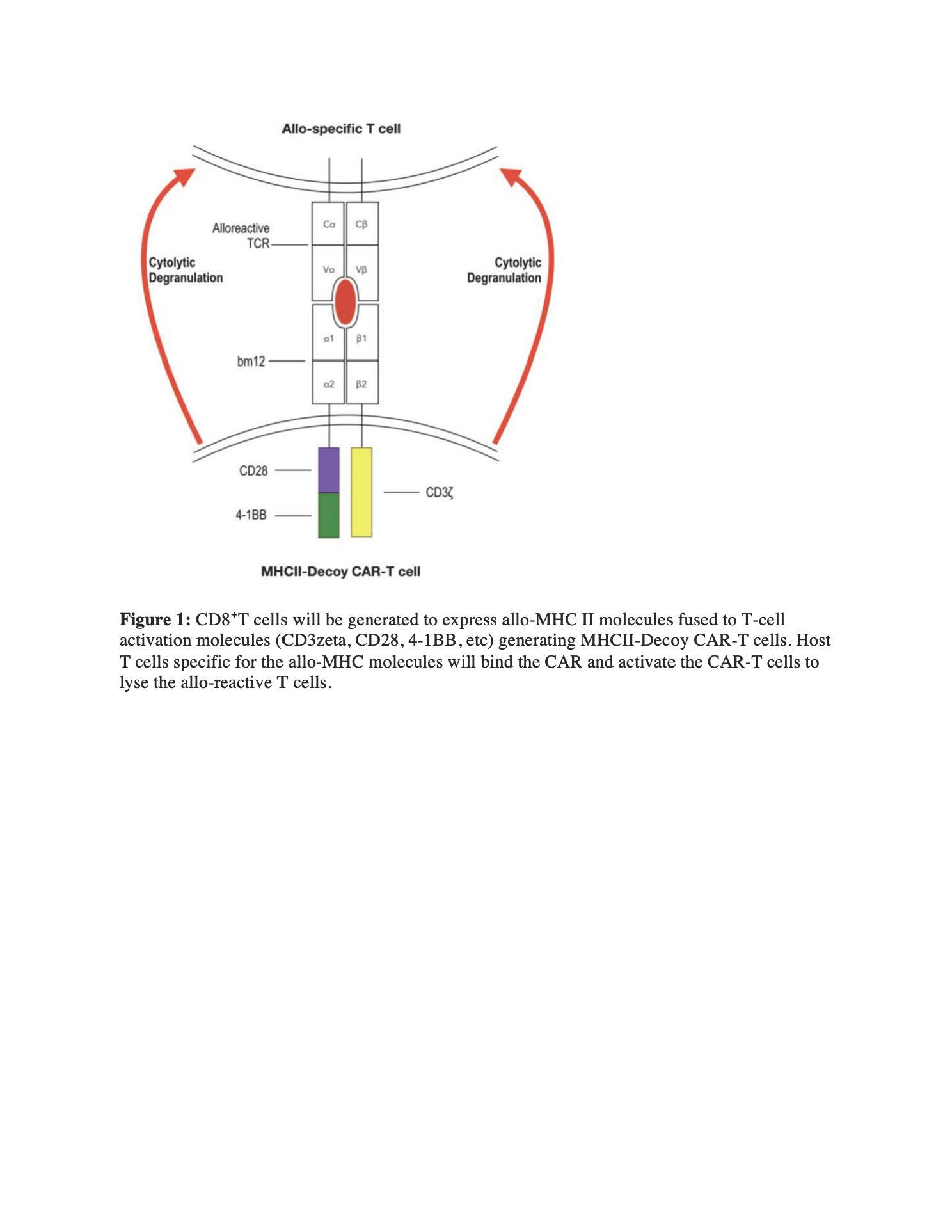
*Purpose: Allotransplantation represents the definitive therapy for end-stage renal, heart, liver, and lung disease. Current immunosuppressive regimens fail to promote long-term engraftment, leading to chronic rejection requiring additional, costly surgical interventions. In spite of immunosuppressive regimens, allotransplant rejection is mediated by host CD4+T cells recognizing donor MHC Class II receptors in allograft tissues, requiring a targeted approach to eliminate these donor-specific CD4+ T cells. Chimeric antigen receptor (CAR)-T cells have demonstrated remarkable efficacy in the treatment of hematological malignancies, using engineered T-cells to selective kill malignant B-cells.
*Methods: We have designed a donor-derived MHC Class II Decoy CAR (Allo-CAR) to selectively deplete host CD4+ T cells expressing T-cell receptors (TCRs) specific for donor Class II. We have designed a bicistronic 3rd generation CAR composed of the C57BL/6 (B6) MHC Class II I-Ab alpha chain coupled through a transmembrane domain to the intracellular signaling domains of CD28 and 4-1BB, followed by a self-cleavable T2A linker and the I-Ab beta chain coupled to CD3ζ. This split design was chosen to promote safety, requiring both the alpha and beta chains to form a productive MHC molecule to transmit the full complement of CAR-mediated T-cell signaling.
*Results: To demonstrate productive cell surface expression of the Allo-CAR, HEK293T and BALB/c T-cells were genetically manipulated and assessed by flow cytometry. However, the Allo-CAR demonstrated low surface expression, suggesting potential instability of the chimeric Class II-based CAR molecule.
*Conclusions: Future experiments will identify stable Allo-CAR systems and testing of Allo-CAR-T cells to eliminate donor-specific T cells and prevent graft rejection in animal models. The Allo-CAR approach could provide an immunosuppression-independent, long-term, self-regulated therapeutic solution for allotransplant rejection.
To cite this abstract in AMA style:
McCall W, Baybutt T, Snook A, Shah A. MHC II-Decoy Car-T Cells to Induce Long-Term Allotransplant Organ Tolerance [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/mhc-ii-decoy-car-t-cells-to-induce-long-term-allotransplant-organ-tolerance/. Accessed February 25, 2026.« Back to 2020 American Transplant Congress