Maribavir for Treatment of Cytomegalovirus Infections Resistant or Refractory to Ganciclovir or Foscarnet in Solid Organ Transplant Recipients: A Phase 2 Study.
M. Pereira,1 F. Silveira,2 G. Papanicolaou,3 A. Langston,4 R. Avery,5 A. Wijatyk,6 J. Wu,6 M. Boeckh,7 F. Marty,8 S. Villano.9
1Columbia University Medical Center, New York, NY
2University of Pittsburgh Medical Center, Pittsburgh, PA
3Memorial Sloan Kettering Cancer Center, New York, NY
4Winship Cancer Institute, Atlanta, GA
5Johns Hopkins University, Baltimore, MD
6Shire, Lexington, MA
7Fred Hutchinson Cancer Research Center, Seattle, WA
8Brigham and Women's Hospital, Boston, MA
9Shire (At Time of Study), Wayne, PA
Meeting: 2017 American Transplant Congress
Abstract number: 562
Keywords: Cytomeglovirus, Recurrence, Safety, Viral therapy
Session Information
Session Time: 8:00am-10:00am
 Presentation Time: 9:00am-9:15am
Presentation Time: 9:00am-9:15am
Location: Arie Crown Theater
Study NCT01611974 assessed safety, tolerability, and antiviral activity of maribavir (MBV) for treatment of resistant or refractory (R/R) CMV infections among transplant recipients. Those aged ≥12 years with ≥1000 DNA copies/mL in blood/plasma, R/R to (val)ganciclovir/foscarnet, were randomized 1:1:1 to oral MBV 400, 800, or 1200 mg BID, for up to 24 weeks. Primary safety analysis focused on incidence of treatment-emergent adverse events (AEs). Primary efficacy endpoint was the proportion of patients with confirmed undetectable plasma CMV DNA within 6 weeks' treatment. In a prespecified subgroup analysis, primary and selected secondary endpoints were evaluated by transplant type. 120 patients were randomized (40/dose group; 73 solid organ transplant [SOT]); median age 55 (range 18–74) years. Efficacy results are shown.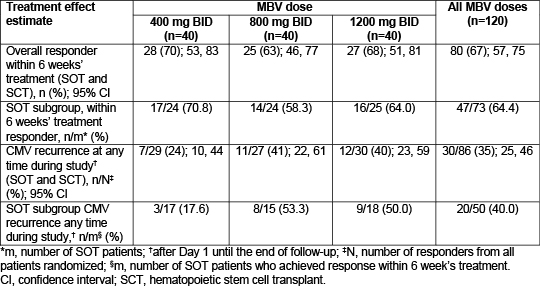 MBV was discontinued owing to an AE in 41/120 (34%) patients; of these, 17/41 were due to CMV infection. MBV dose-adjustments occurred in 15/120 (13%) patients and AEs causing drug interruptions were reported in 20/120 (17%) patients. Neutropenia(ANC <1000/mm3) occurred in 12/106 (11%) patients at any time during the study (baseline 17/106 [16%]); rates were similar across doses. In SOT, MBV 400–1200 mg BID was effective for treatment of CMV infection R/R to prior therapy. Data support the safety of MBV administered for up to 24 weeks and are consistent with previous trials, with no evidence of myelosuppression above baseline. Phase 3 trials are warranted.
MBV was discontinued owing to an AE in 41/120 (34%) patients; of these, 17/41 were due to CMV infection. MBV dose-adjustments occurred in 15/120 (13%) patients and AEs causing drug interruptions were reported in 20/120 (17%) patients. Neutropenia(ANC <1000/mm3) occurred in 12/106 (11%) patients at any time during the study (baseline 17/106 [16%]); rates were similar across doses. In SOT, MBV 400–1200 mg BID was effective for treatment of CMV infection R/R to prior therapy. Data support the safety of MBV administered for up to 24 weeks and are consistent with previous trials, with no evidence of myelosuppression above baseline. Phase 3 trials are warranted.
CITATION INFORMATION: Pereira M, Silveira F, Papanicolaou G, Langston A, Avery R, Wijatyk A, Wu J, Boeckh M, Marty F, Villano S. Maribavir for Treatment of Cytomegalovirus Infections Resistant or Refractory to Ganciclovir or Foscarnet in Solid Organ Transplant Recipients: A Phase 2 Study. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Pereira M, Silveira F, Papanicolaou G, Langston A, Avery R, Wijatyk A, Wu J, Boeckh M, Marty F, Villano S. Maribavir for Treatment of Cytomegalovirus Infections Resistant or Refractory to Ganciclovir or Foscarnet in Solid Organ Transplant Recipients: A Phase 2 Study. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/maribavir-for-treatment-of-cytomegalovirus-infections-resistant-or-refractory-to-ganciclovir-or-foscarnet-in-solid-organ-transplant-recipients-a-phase-2-study/. Accessed February 17, 2026.« Back to 2017 American Transplant Congress
