Longitudinal Quality of Life Assessment in Liver Transplant – Feasibility Study and Early Results
Northwestern University, Chicago, IL
Meeting: 2021 American Transplant Congress
Abstract number: 1091
Keywords: Liver, Liver cirrhosis, Liver transplantation, Quality of life
Topic: Clinical Science » Liver » Liver: Cirrhosis - Portal Hypertension and Other Complications
Session Information
Session Name: Liver: Cirrhosis - Portal Hypertension and Other Complications?
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: In addition to survival, quality of life (QoL) is critical to optimizing care in liver transplantation (LT). Although QoL is associated with clinical outcomes in other settings, QoL assessments are not part of routine transplant care, and little is known about longitudinal changes pre- and post- LT. Furthermore, assessments performed by clinic staff can be time-consuming and cost prohibitive. Therefore, we aimed to measure QoL in pre- and post LT patients via brief monthly self-assessments.
*Methods: A prospective single-center study included patients with cirrhosis evaluated for LT 1/2018 – 10/2020; both pre- and post- LT patients were included. Recruitment began in 10/2020 and is ongoing. The NIH Patient Reported Outcomes Measurement Information System (PROMIS) is used to measure QoL through online computerized adaptive tests (CATs) emailed to patients at baseline and repeated monthly. All PROMIS measures are reported as t scores referenced to the general US population with a mean of 50 and an SD +/- 10. A score above 50 indicates that a subject has a higher degree of the domain relative to the general US population.
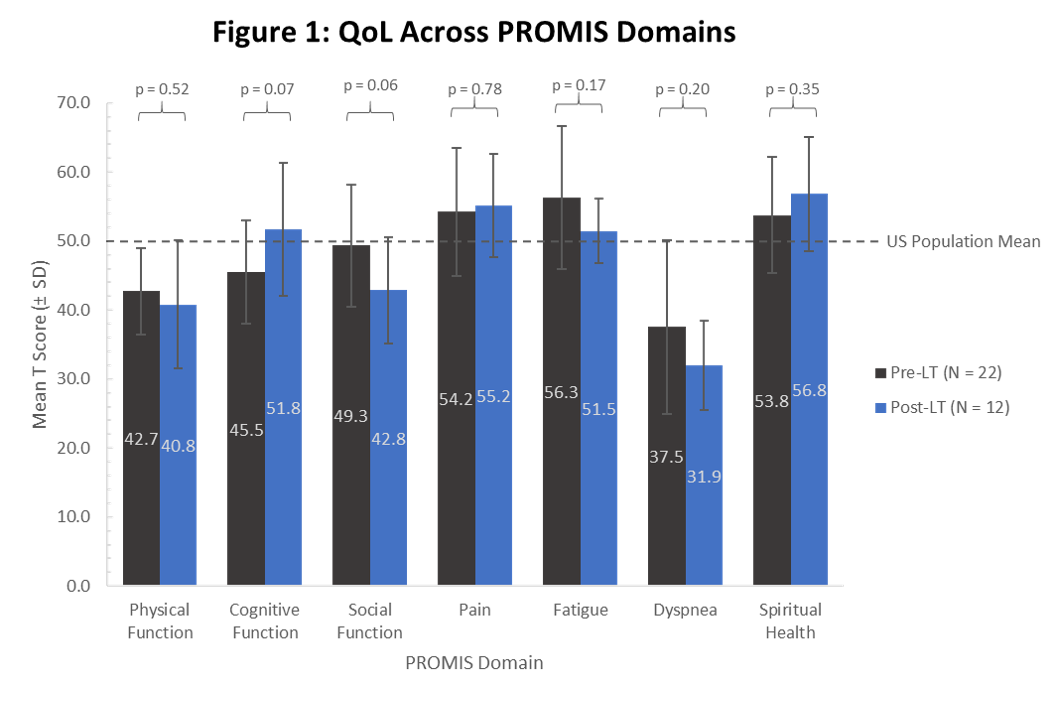
*Results: 39 patients were enrolled from 10/14 to 11/19 2020 [expected enrollment by 6/21 (ATC 2021) is 135]. 87% of patients completed all baseline surveys, 36% were female, 82% were White, 3% Black and 13% Hispanic. Etiology of cirrhosis was NASH (41%), ETOH (28%), HCV/HBV (18%), Biliary (13%), and Other (23%). 27 (69%) were pre-LT with a mean MELD 14.9 +/- 5.5 and 12 (31%) were post-LT with mean time since transplant of 1.9 months (range 0-5). The sample is not yet powered to show significance, but trends in the pre vs post LT comparison show an increase in perceived cognitive function (+6.3), and reduction of dyspnea (-5.6) and fatigue (-4.8), as well as increased spiritual health (+ 3). Pre/post LT physical function remains below the general population (42.7/40.8) while pain remains high (54.2/55.2) and social function drops (49.3/42.8). (Figure 1)
*Conclusions: PROMIS CATs can successfully be used to measure QoL in pre- and post- LT patients via online self-assessments without assistance by a coordinator. Early results show high response rates (87%) and trends in changes pre- and post- LT, which are promising as enrollment continues. Change in QoL domain scores over time and association with adverse clinical outcomes will be examined as monthly data is collected. Thus, PROMIS CATs may provide a cost-effective way to longitudinally assess QoL in LT patients and optimize outcomes.
To cite this abstract in AMA style:
Siddiqui OM, Polineni P, Thuluvath AJ, Loftus C. Longitudinal Quality of Life Assessment in Liver Transplant – Feasibility Study and Early Results [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/longitudinal-quality-of-life-assessment-in-liver-transplant-feasibility-study-and-early-results/. Accessed February 18, 2026.« Back to 2021 American Transplant Congress