Long Term Outcomes of Tocilizumab Therapy for DSA+ Antibody Mediated Rejection (ABMR) Resistant to IVIG + Rituxan (I+R) Treatment
1CTC, Cedars Sinai, Los Angeles, CA
2Pathology, Cedars-Sinai, Los Angeles, CA
3HLA Laboratory, Cedars-Sinai, Los Angeles, CA.
Meeting: 2015 American Transplant Congress
Abstract number: 95
Keywords: B cells, HLA antibodies, Kidney transplantation, Rejection
Session Information
Session Name: Concurrent Session: Kidney Antibody Mediated Rejection II
Session Type: Concurrent Session
Date: Sunday, May 3, 2015
Session Time: 4:00pm-5:30pm
 Presentation Time: 5:00pm-5:12pm
Presentation Time: 5:00pm-5:12pm
Location: Terrace I-III
Intro: Limited options are available for treatment of ABMR in highly-HLA sensitized patients (HS). Treatment options include I+R, PLEX+I+/- R, C5-inhibitor & bortezomib. Approximately 25% of HS patients are at risk for ABMR+ with development of chronic TG & return to dialysis. Emerging data suggest IL-6 may have an important role in ABMR+ injury & inflammation that occurs post-tx. Here, we examined the long term use of TCZ for treatment of ABMR+/DSA+ (+/- angiotensin-1 receptor (AT1R) antibody (ab) patients unresponsive to treatment with I+R or PLEX+I+R. Methods: From 4/2011-11/2014, we identified 23 ABMR+ patients including those with chronic TG, DSA+ and/or AT1R ab+. Briefly, patients received TCZ at 4-8mg/kg, monthly X 3-11M. Patients were monitored for DSA and renal function. AT1R ab results were also monitored in 7/23 (30%) patients. Results: 21/23 (91%) patients had significant pathologic findings of ABMR (17/21 (81%) patients with TG) usually with elevated DSA levels and/or elevated AT1R ab at time of treatment. After TCZ treatment, DSA levels decreased in 10/23 patients (43%) while 6/23 (26%) remained stable at 18M. One patient had significant rebound after TCZ was discontinued. 4/6 (67%) patients with stable DSA scores had C1Q positive DSAs, 3 patient had both C1Q+ DSA & AT1R ab+ & 1 TG patient had no identifiable antibodies. SAEs included, 2 patients had TCZ postponed after 4th dose d/t temporary vision loss & eye numbness, 1 patient had dose adjusted for neutropenia. Renal function was stable in 21/23 patients (91%) while one patient lost the allograft due to unremitting ABMR+. One patient with NSF showed significant improvement in skin softening with stable Cr & improved DSA-RIS. Conclusions: From our single center experience, TCZ continues to show promising results in ABMR+ patients resistant to PLEX +/- I + R. Potential benefits of TCZ therapy include inhibition of B-cell activation, reduction in plasma cell Ig production and induction of Tregs.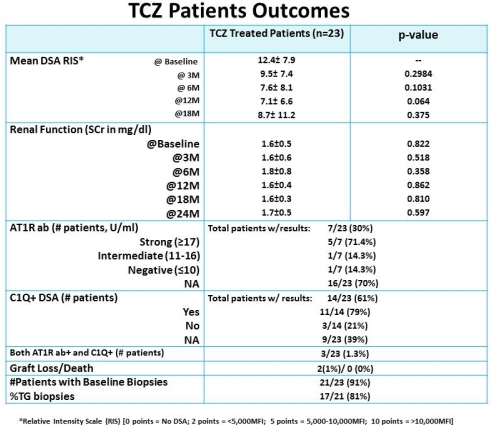
To cite this abstract in AMA style:
Choi J, Vo A, Kahwaji J, Haas M, Reinsmoen N, Puliyanda D, Peng A, Villicana R, Jordan S. Long Term Outcomes of Tocilizumab Therapy for DSA+ Antibody Mediated Rejection (ABMR) Resistant to IVIG + Rituxan (I+R) Treatment [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-outcomes-of-tocilizumab-therapy-for-dsa-antibody-mediated-rejection-abmr-resistant-to-ivig-rituxan-ir-treatment/. Accessed March 9, 2026.« Back to 2015 American Transplant Congress
