Long-Term Outcomes After Conversion to a Belatacept-Based Immunosuppression in Kidney Transplant: A Matched Cohort Study
Paris Transplant Group, INSERM, Paris, France
Meeting: 2022 American Transplant Congress
Abstract number: 414
Keywords: Calcineurin, Co-stimulation, Graft survival, Immunosuppression
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 4:20pm-4:30pm
Presentation Time: 4:20pm-4:30pm
Location: Hynes Room 302
*Purpose: Conversion to belatacept after transplant seems to be safe. However, no studies have reported the long-term efficacy and safety outcomes after conversion to belatacept and compared it with patients treated with a CNI based regimen in kidney transplantation. This study aims to investigate the long-term outcomes of patients converted to belatacept compared with matched patients under a CNI regimen.
*Methods: Kidney transplant recipients transplanted between 1998 and 2019 from two French academic transplant canters were recruited. We used a propensity score to match with a 1:1 ratio, patients at time of the biopsy which indicate the conversion to belatacept to control patients under a CNI regimen with a biopsy after transplant. We used 11 parameters associated with graft survival for the matching, 4 baselines transplant characteristics (recipient age, prior transplant status, donor type, dgf) and 7 at time of the biopsy (time after transplant, eGFR, proteinuria, DSA and Banff scores cv, ah, IFTA). Transplant outcomes defined by graft and patient survival, as well as safety outcomes were compared between the matched patients.
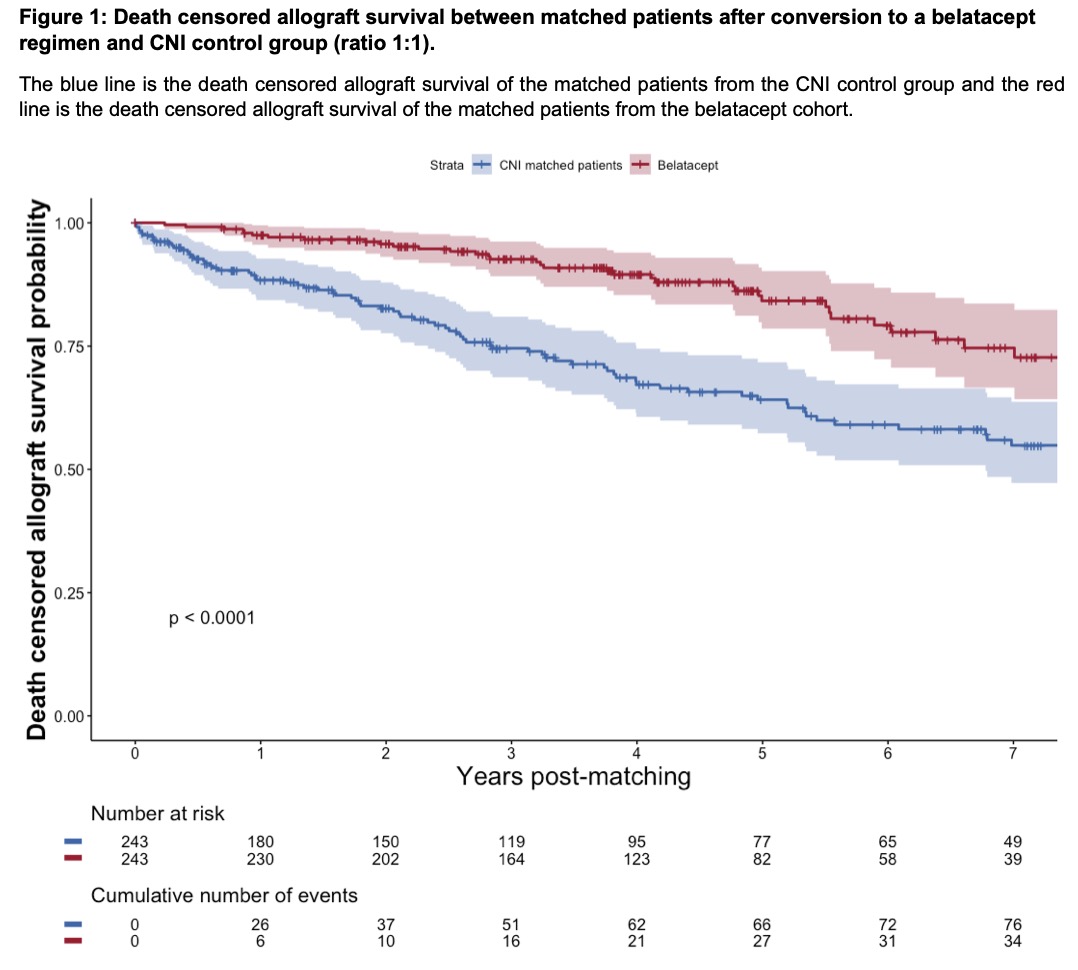
*Results: From 3215 kidney transplants recipients transplanted during the study period with the inclusion criteria (311 patients under belatacept and 2904 patients under CNI). A total of 243 patients under belatacept were matched with 243 controls under CNI. All prognostic parameters were well balanced before conversion between the 2 arms with a mean age of 54.7 ±15.1 (p=0.4543), mean time of 2.2 ±3.2 years after transplant (p=0.586), mean eGFR of 33.0 ±13.3 (p=0.976) in the belatacept group. After a mean follow-up time after conversion of 4.4 ± 2.5 years, 36 (14.8%) patients lost their graft and 39 (16.0%) patients died in the belatacept arm. After conversion to a belatacept regimen, the graft survival was significantly improved when we compared with the matched patients’ under CNI p<0.0001 (Figure 1). Patients converted to belatacept showed lower death rate of 16% compared with 30% for the CNI treated patients (p<0.001). The safety outcomes show similar rate of biopsy proven rejection, major adverse cardiovascular events, and cancer between the 2 groups, while a significant higher rate of CMV disease was observed among the belatacept treated patients p<0.01).
*Conclusions: This study confirms that conversion to belatacept post-transplant is associated with improved long term graft outcome and acceptable safety. Conversion to belatacept after transplant should be considered as a valuable therapeutic option in kidney transplant recipients.
To cite this abstract in AMA style:
Divard G, Debiais C, Legendre C, Aubert O, Lefaucheur C, Loupy A. Long-Term Outcomes After Conversion to a Belatacept-Based Immunosuppression in Kidney Transplant: A Matched Cohort Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/long-term-outcomes-after-conversion-to-a-belatacept-based-immunosuppression-in-kidney-transplant-a-matched-cohort-study/. Accessed March 2, 2026.« Back to 2022 American Transplant Congress