Left Ventricular Assist Device–Associated Allosensitization — Clinical Implications
U.T.A.H. Cardiac Transplant Program, Salt Lake City, UT.
Meeting: 2015 American Transplant Congress
Abstract number: 71
Keywords: Alloantibodies, Rejection, Sensitization, Ventricular assist devices
Session Information
Session Name: Concurrent Session: "The Pit and the Pendulum": VADs, Dual Organs and Other Matters of the Heart
Session Type: Concurrent Session
Date: Sunday, May 3, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 2:39pm-2:51pm
Presentation Time: 2:39pm-2:51pm
Location: Room 119-B
Purpose: Allosensitization has been recognized as a prognostic marker of worse outcome after transplant. It is unclear, however, whether sensitization events that take place shortly before transplant have a different prognostic value than sensitization due to other more remote etiologies.
Methods: Alloantibodies were detected with single-antigen beads on Luminex platform in all patients who received heart transplant from 2006 to 2014. Allosensitization was defined as cPRA >10%. In pts receiving CF LVADs as a bridge to transplant, cPRA was checked before LVAD implantation and at the time of transplant. In pts who had transplants without LVAD, cPRA was checked at the time of listing and at the time of transplant. Antibody mediated rejection was defined as 3 or more episodes of pAMR≥2 detected on endomyocardial biopsies.
Results: 197 pts were transplanted between 2006 and 2014, of which 78 (40%) were bridged with CF LVADs. In this group (BTT CF LVAD), 9 (11.5%) were sensitized prior to LVAD implantation. Among the remaining pts (Transplant without LVAD), 13 (10.9%) were sensitized at the time of listing. The incidence of de novo sensitization while awaiting transplantation was similar in the two groups – 5 (6.4%) in the BTT CF LVAD group with cPRA range of 16-24% vs. 12 (5.9%) in the Transplant without LVAD group with cPRA range of 23-97%, p=NS. De novo sensitization associated with LVAD implant was strongly associated with AMR (p<0.001, Figure 1). This association was not seen, however, in pts without LVADs (p=0.53, Figure 1).
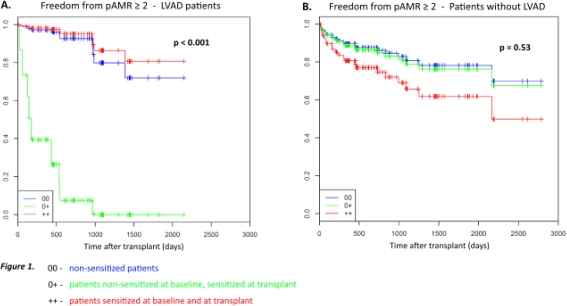
Conclusions: De novo sensitization occurred both in patients who were bridged with CF LVADs and in patients who had transplants without LVADs while awaiting transplant. Patients in whom sensitization was triggered by CF LVAD implant had a high risk of AMR after transplant. Different immunosuppression and surveillance strategies might need to be considered for this group of patients.
To cite this abstract in AMA style:
Ko B-S, Willis C, Drakos S, Hurst D, Kfoury A, Snow G, Delgado J, Hammond E, Selzman C, Alharethi R, McKellar S, Nativi-Nicolau J, Gilbert E, Revelo P, Miller D, Reid B, Fang J, Eckles D, Stehlik J. Left Ventricular Assist Device–Associated Allosensitization — Clinical Implications [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/left-ventricular-assist-deviceassociated-allosensitization-clinical-implications/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
