Is it Time for Immune Screening for Belatacept-Treated Stable Kidney Transplant Patients for Adjust the Immunosuppression?
1ITAC, Buenos Aires, Argentina, 2CEFYBO, UBA, Buenos Aires, Argentina
Meeting: 2020 American Transplant Congress
Abstract number: A-330
Keywords: Co-stimulation, Immunosuppression, Monitoring, T cell activation
Session Information
Session Name: Poster Session A: Biomarker Discovery and Immune Modulation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Belatacept is a co-stimulation blocker for primary maintenance immunosuppression in kidney transplant recipients. The goal of this work was to study the immunological status of stable kidney transplant patient that receive belatacept in order to unravel whether belatacept dose should be adjusted in each patient based on immune cell phenotype parameters.
*Methods: A total of 15 patients, with at least 9 month from transplant were enrolled after giving informed consent according to the Declarations of Helsinki. The protocol was approved by an Ethic Committee. Three samples of peripheral blood were taken for each patient: twice just before the regular dose of belatacept (day 28th) and one at 14th day after the dose (day 14th). Several costimulatory molecules (CD80, CD86, B7H2, CD40, 4-1BBL, CD70, OX40L, PD-L1, PD-L2) expression were assessed on professional antigen presenting cells (monocytes and B cells) and their ligands on T cells (CD28, CTLA-4, PD-1, ICOS, SLAM, CD25, CD27, CD137, CD40L, OX40) by flow cytometry.
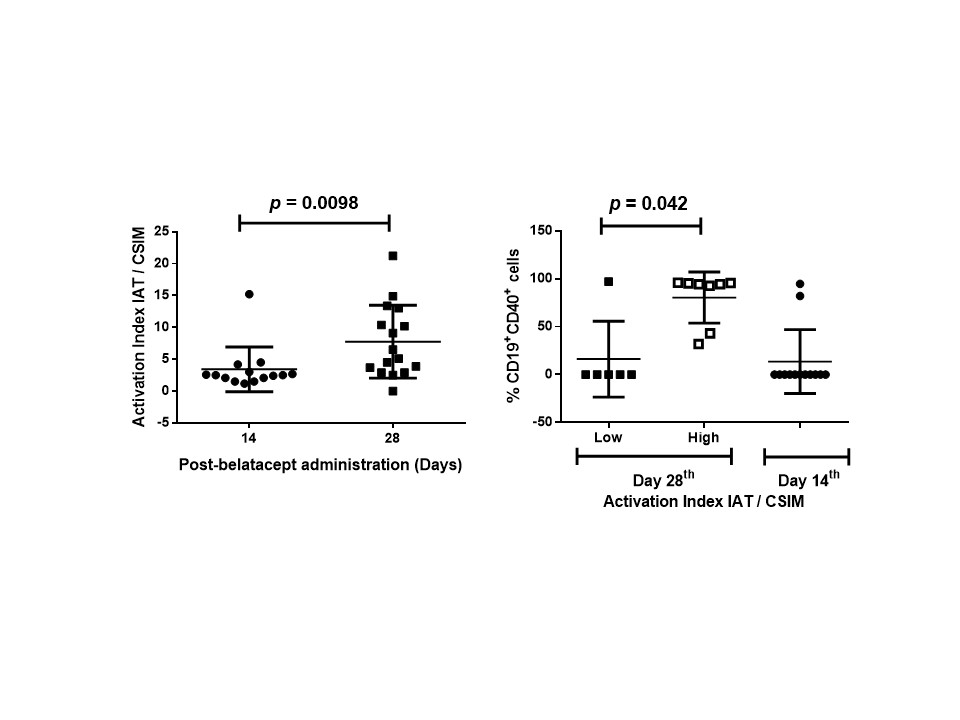
*Results: Overall, the expression of costimulatory signals and their ligands showed interpatient variability in most of the molecules analyzed. To analyze costimulatory and co-activation or -inhibition signals for each patient, we generated several index named costimulatory index for monocytes (CSIM), costimulatory index for B cells (CSIB) and an activation index for T cells (AIT). Surprisingly, the CSIM was higher in the samples taken at 14th day than 28th day after regular dose of belatacept. On the contrary, the AIT and the CSIB was higher in the samples taken at 28th than 14th day after the regular dose of belatacept. The relationships between AIT and CSIB showed that all patients had higher index at 28th day than 14th day. However, the AIT/CSIM index of day 28th revealed two groups of patients; those with similar costimulatory and activation signals than samples taken at 14th day after belatacept treatment and those with high costimulatory and activation signals (Figure 1). The only marker that distinguished the patients with high costimulatory and activation signals at day 28th from the rest of the patients was the presence of CD19+CD40+ cells (Figure 2).
*Conclusions: Based on these results and considering that immune response activation at day 14th should be lower than on day 28th after belatacept treatment, the data described herein suggest that the dose could be adjust for each patient based on the AIT/CSIM index or the assessment of CD19+CD40+ cells just before regular dose of belatacept.
To cite this abstract in AMA style:
Rial MC, Caro F, Ambrosi N, Guerrieri D, Calleri A, Chuluyan E, Casadei D. Is it Time for Immune Screening for Belatacept-Treated Stable Kidney Transplant Patients for Adjust the Immunosuppression? [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/is-it-time-for-immune-screening-for-belatacept-treated-stable-kidney-transplant-patients-for-adjust-the-immunosuppression/. Accessed July 18, 2025.« Back to 2020 American Transplant Congress