iNKT Cell Ligands Administered with Bone Marrow Cells Expand Allo-Specific Regulatory T Cells In Vivo and Establish Transplant Tolerance.
1Urology, Tokyo Women's Medical University, Tokyo, Japan
2Cardiovascular Surgery, Tokyo Women's Medical University, Tokyo, Japan
3Vaccine Design, RIKEN IMS-RCAI, Yokohama, Japan.
Meeting: 2016 American Transplant Congress
Abstract number: D36
Keywords: Bone marrow, Donor specific transfusion, Engraftment, Graft survival
Session Information
Session Name: Poster Session D: Chimerism/Stem Cells, Cellular/Islet Transplantation, Innate Immunity, Chronic Rejection
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Invariant natural killer T(iNKT) cells have the powerful potential to expand regulatory T cells(Tregs), especially when they are presented α-galactosylceramide(aGC) by B cells. We previously reported that a liposomal αGC(lipo-aGC) was preferentially incorporated into B cells rather than dendritic cells, and induced ovalbumin(OVA)-specific Tregs in vivo when administered with OVA. In the current study, we administered lipo-aGC with allogeneic cells, such as bone marrow cells(BMCs) and splenocytes(SPCs), to evaluate whether lipo-aGC regulates allo-immune responses through allo-specific Treg generation.
3Gy-irradiated BALB/c(H2d) mice were transferred BMCs or SPCs obtained from allogeneic donor(B6; H2b), and subsequently injected lipo-aGC. To support cell engraftment, 0.5mg anti-CD40L mAb was also injected. Both BMCs and SPCs were engrafted on day7 after transfer. Interestingly, only the BMC-transferred mice showed expansion of highly proliferating Tregs in the spleen on day7, whereas the SPC-transferred mice did not(Fig 1). To evaluate antigen specificity of Tregs, we adoptively transferred H2d+CD4+CD25+Tregs isolated from the BMC-transferred mice on day7 into SCID mice that were transplanted skin grafts from B6 and C3H(H2k) mice simultaneously. Thereafter, naive BALB/c T cells were administered into the SCID mice. Although both B6- and C3H-derived graft survival was prolonged, C3H-derived graft was rejected earlier than that derived from B6, suggesting that the Tregs expanded by lipo-aGC plus BMC-transfer have BM donor-specific suppressive potential. Furthermore, when heart transplant was performed on the next day of BMC-transfer, the mice accepted BM donor-derived heart allograft, whereas they rejected the graft from 3rd party donor(Fig 2). The SPC-transferred mice rejected heart allograft even though the graft was derived from the same donor. Taken together, we presume that lipo-aGC generates allo-specific Tregs in vivo when co-administrated with allogeneic BMCs.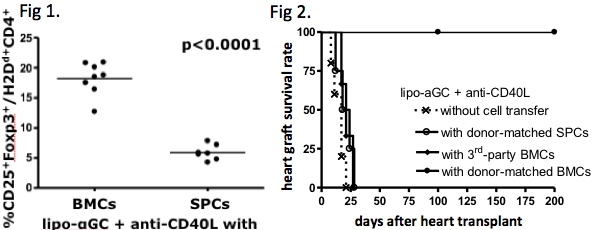
CITATION INFORMATION: Miyairi S, Hirai T, Okumi M, Ishii Y, Yamazaki K, Tanabe K. iNKT Cell Ligands Administered with Bone Marrow Cells Expand Allo-Specific Regulatory T Cells In Vivo and Establish Transplant Tolerance. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Miyairi S, Hirai T, Okumi M, Ishii Y, Yamazaki K, Tanabe K. iNKT Cell Ligands Administered with Bone Marrow Cells Expand Allo-Specific Regulatory T Cells In Vivo and Establish Transplant Tolerance. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/inkt-cell-ligands-administered-with-bone-marrow-cells-expand-allo-specific-regulatory-t-cells-in-vivo-and-establish-transplant-tolerance/. Accessed February 22, 2026.« Back to 2016 American Transplant Congress
