Initial Experience with the Bacterial Enzyme IdeS® (IgG Endopeptidase) for Desensitization of Highly-HLA Sensitized (HS) Patients.
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2Pathology, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2016 American Transplant Congress
Abstract number: D307
Keywords: B cells, Highly-sensitized, HLA antibodies
Session Information
Session Name: Poster Session D: Late Breaking
Session Type: Poster Session
Date: Tuesday, June 14, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Introduction: The IgG degrading enzyme derived from Streptococcus pyogenes (IdeS®) is a 35k-Da cysteine protease that cleaves human IgG molecules at the lower hinge region creating one F(ab)'2 and one homodimeric Fc fragment, thus inhibiting CDC and ADCC. IdeS also cleaves the IgG-type B-cell receptor (BCR) completely abrogating IgG-B-cell memory responses in vitro. This suggests that IdeS could be useful for DES of HS patients. Recent data show IdeS can reduce all IgG molecules to fragments within 4-6 hrs. Here, we report our experience with IdeS® for DES to accomplish incompatible transplantation. Methods & Patients: This is an initial evaluation of our FDA approved study (IND124301 NCT 02426684). From 6/15-1/16, 4 patients entered the trial. Three of 4 patients had numerous DSAs and +FCMX. cPRAs ranged (92->100%). All received 0.24mg/Kg IdeS IV 4-6 hrs prior to incompatible DD kidney transplant. All had frequent measurement of DSAs post-transplant and renal allograft biopsies within the first 2 weeks post-transplant. Results: IdeS treatment was not associated with discernable infusion-related side effects. Assessment of DSAs beginning 6 hrs post-transplant showed complete DSA elimination (>2M in one patient). Patients also received IVIG & rituximab ~ 10 days post-IdeS. Results of post-transplant biopsies showed evidence of ATN in 3/4 while one patient had Banff 1B CMR which responded well to pulse steroid treatment. No patient had evidence of ABMR. One patient had mild ABMR at 4M post-IdeS that responded to treatment. DSA data pre/post-IdeS is shown in figure 1 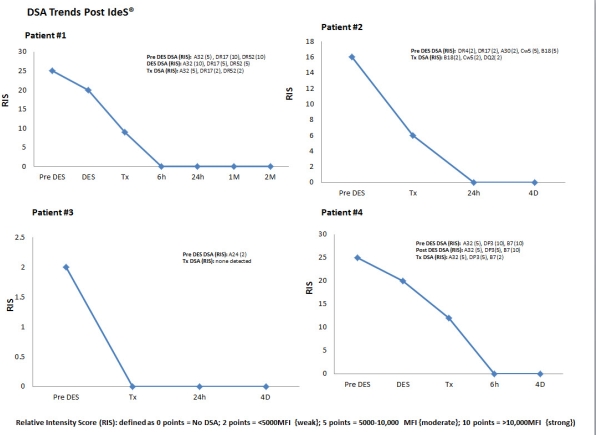 . Conclusions: 1) IdeS treatment of HS patients completely eliminates DSAs present at transplant and allows for successful transplantation of HLA incompatible patients. 2) IdeS is well tolerated without infusion-related side effects and no significant infections to date. 3) IdeS may provide a rapid, effective and durable method to eliminate DSAs and transplant HS patients who are resistant to current DES.
. Conclusions: 1) IdeS treatment of HS patients completely eliminates DSAs present at transplant and allows for successful transplantation of HLA incompatible patients. 2) IdeS is well tolerated without infusion-related side effects and no significant infections to date. 3) IdeS may provide a rapid, effective and durable method to eliminate DSAs and transplant HS patients who are resistant to current DES.
CITATION INFORMATION: Jordan S, Choi J, Zhang X, Haas M, Peng A, Kahwaji J, Villicana R, Vo A. Initial Experience with the Bacterial Enzyme IdeS® (IgG Endopeptidase) for Desensitization of Highly-HLA Sensitized (HS) Patients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Jordan S, Choi J, Zhang X, Haas M, Peng A, Kahwaji J, Villicana R, Vo A. Initial Experience with the Bacterial Enzyme IdeS® (IgG Endopeptidase) for Desensitization of Highly-HLA Sensitized (HS) Patients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/initial-experience-with-the-bacterial-enzyme-ides-igg-endopeptidase-for-desensitization-of-highly-hla-sensitized-hs-patients/. Accessed February 25, 2026.« Back to 2016 American Transplant Congress
