Infections in Kidney Transplant Recipients on Belatacept versus Tacrolimus-Based Immunosuppression
1Emory Healthcare, Atlanta, GA, 2Emory University Hospital, Atlanta, GA
Meeting: 2022 American Transplant Congress
Abstract number: 1351
Keywords: Cytomeglovirus, Infection, Kidney, Polyma virus
Topic: Clinical Science » Infection Disease » 26 - Kidney: Polyoma
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Patients 70 years and older with advanced/ end stage kidney disease are increasingly receiving kidney transplants. Immediately post- transplant, our center utilizes Belatacept coupled with an 11-month tacrolimus overlap period, mycophenolate (MMF), and prednisone. CMV High risk Kidney Transplant Recipients(KTRs) are placed on tacrolimus based regimen per our center’s policy at time of transplant. Amongst KTRS, the risk of infections and associated death proportionately increases with advancing age. In older KTRs it is unknown if being on 4 immunosuppression agents itself increases risks of CMV and BK viremia ultimately resulting in poor patient and allograft outcomes. We assessed the incidence of CMV and BK viremia and compared differences in allograft function and number of hospitalizations one year post transplant in older KTRs on belatacept versus tacrolimus-based regimens.
*Methods: We performed a single center retrospective analysis amongst KTRs, 70 years or older at time of transplant, who were transplanted at our center between January 31 2017 to August 31st 2020. KTRs with less than 1 year of follow-up or those converted from one immunosuppression regimen to another were excluded from the study. CMV Viremia was defined as CMV PCR >35 IU/ml and BK Viremia was defined as BK DNA PCR of ≥ 3.5 log 10 copies/mL. Using t-tests, we compared 2 groups based on immunosuppression regimen: Belatacept and Tacrolimus.
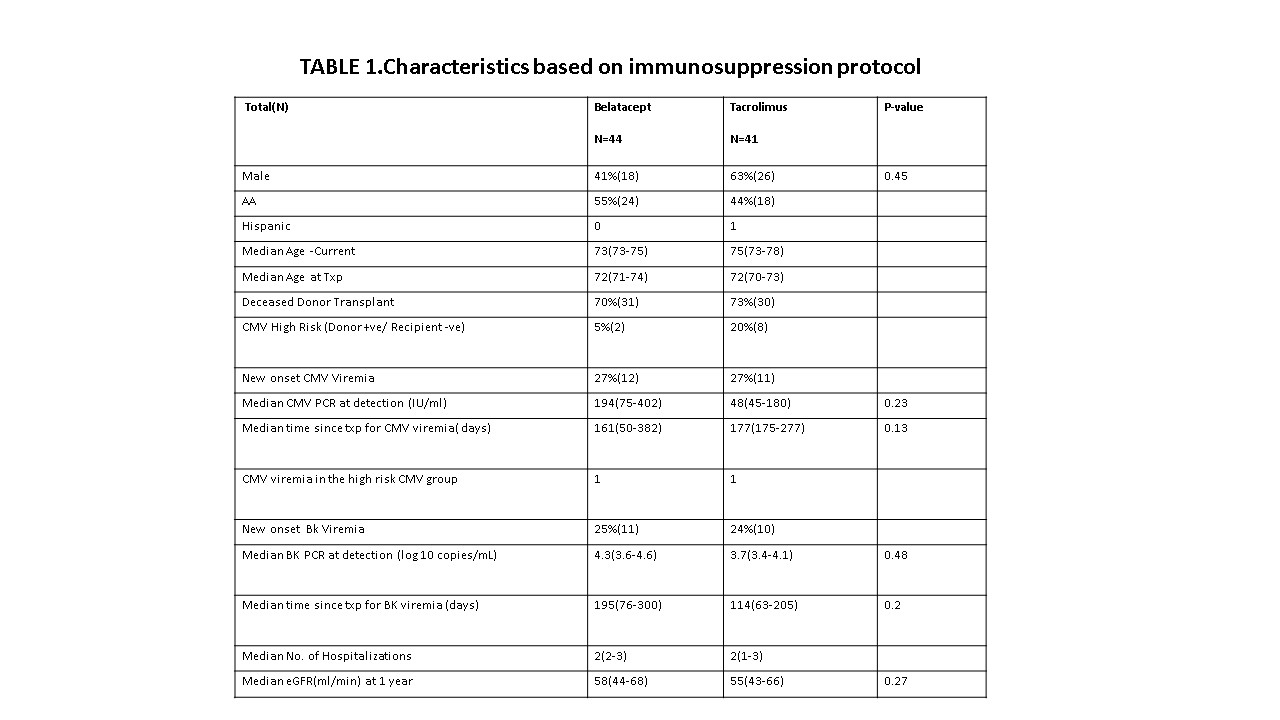
*Results: 85 patients were included in this study: Belatacept (n=44), Tacrolimus (n=41). Both groups were similar in their demographics. (TABLE1). There was no significant difference in the incidence of CMV Viremia (27% Vs 27%) and median CMV PCR at time of detection (198 Vs 48, p 0.23) between the two groups. CMV high risk patients were more likely to be on a tacrolimus based regimen (2 Vs 8). 50% (1/2) of CMV High Risk patients in the Belatacept cohort developed CMV Viremia. BK Viremia incidence (25% Vs 24%) and BK DNA PCR (4.3 Vs 3.7, p 0.48) were similar in both cohorts. Both groups had similar number of hospitalizations and renal function at one year post transplant. No deaths or allograft loss was observed in either groups.
*Conclusions: Belatacept can safely be used in all eligible older KTRs except for those at high risk for developing CMV viremia in whom careful risk versus benefit assessment is recommended.
To cite this abstract in AMA style:
Gattis S, Basu A. Infections in Kidney Transplant Recipients on Belatacept versus Tacrolimus-Based Immunosuppression [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/infections-in-kidney-transplant-recipients-on-belatacept-versus-tacrolimus-based-immunosuppression/. Accessed February 21, 2026.« Back to 2022 American Transplant Congress