Immunogenicity and Safety of SARS-CoV-2 Vaccines and Breakthrough Infections Among Solid Organ Transplant Recipients: Systematic Review and Meta-Analysis
A. Villavicencio1, Y. Ebisu2, M. Raja3, A. P. Sanchez-Covarrubias1, S. Anjan3, J. M. Reynolds1, J. Simkins3, J. Camargo3, M. I. Morris3, L. Abbo4, G. Guerra5, Y. Natori6
1Internal Medicine, University of Miami Miller School of Medicine, Miami, FL, 2Kameda Medical Center, Chiba, Japan, 3University of Miami Miller School of Medicine, Miami, FL, 4University Of Miami Miller School of Medicine, Miami, FL, 5University of Miami Jackson Memorial Hospital, Miami, FL, 6University of Miami, Pinecrest, FL
Meeting: 2022 American Transplant Congress
Abstract number: 982
Keywords: COVID-19, Immunogenicity, Safety, Vaccination
Topic: Clinical Science » Infection Disease » 24 - All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: All Infections (Excluding Kidney & Viral Hepatitis) II
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: The SARS-CoV-2 pandemic has had a significant impact on the field of solid organ transplant(SOT). Immunization against SARS-CoV-2 is globally available since 2021. SOT recipients represent a vulnerable group with a higher risk of infection and worse outcomes from COVID-19 compared with the general population. There is a concern for the efficacy of SARS-CoV-2 vaccination amongst SOT recipients. We aimed to assess immunogenicity, safety and breakthrough infections after SARS-CoV-2 vaccination.
*Methods: We conducted a systematic review and a meta-analysis using articles from 8 databases published from January 1,2020 to July 13,2021. We included studies reporting data regarding SOT and SARS-CoV-2 post vaccine antibody response or cellular response; safety of vaccination; and SARS-CoV-2 infection after at least one vaccine dose. A meta-analysis of postvaccine antibody response and death in breakthrough infections was conducted using a random-effects model.
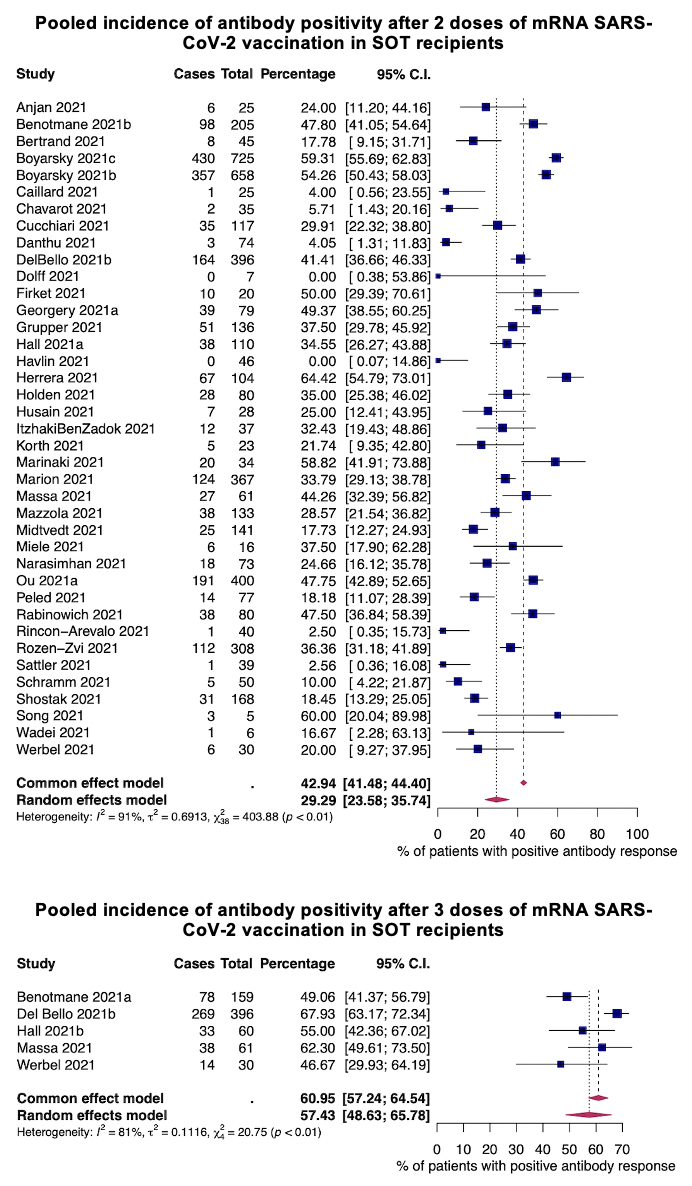
*Results: Initially, we identified 572 potential studies. After careful review, we included 64 studies for systematic review and 46 studies for meta-analysis. We identified 6,710 SOT recipients. Pooled incidence of antibody positivity after completion of any vaccine schedule was 28.3% (95% confidence interval[CI] 22.5-34.8%). Pooled incidence of antibody positivity after messenger RNA vaccination with 2 doses and 3 doses were 29.3%(95%CI 23.58%-35.74%) and 57.4%(95%CI 48.63-65.78%), respectively. Twelve reports on interferon-γ response to SARS-CoV-2 spike antigen peptides showed a positivity between 30.4% and 55.0% after messenger RNA vaccines. The most common side effect after vaccination was site pain. Only 5 cases developed rejection but no graft loss. The pooled incidence of death in breakthrough infections was 17.1%(95%CI 10.2%-27.2%).
*Conclusions: Our findings show that only 29% of SOT recipients could mount antibodies after 2 doses of messenger RNA vaccines, with an improved response seen after 3 doses (57%). Even with 3 doses, the immunogenicity is still suboptimal and further studies to investigate the optimal vaccination strategies in this population are needed.
To cite this abstract in AMA style:
Villavicencio A, Ebisu Y, Raja M, Sanchez-Covarrubias AP, Anjan S, Reynolds JM, Simkins J, Camargo J, Morris MI, Abbo L, Guerra G, Natori Y. Immunogenicity and Safety of SARS-CoV-2 Vaccines and Breakthrough Infections Among Solid Organ Transplant Recipients: Systematic Review and Meta-Analysis [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/immunogenicity-and-safety-of-sars-cov-2-vaccines-and-breakthrough-infections-among-solid-organ-transplant-recipients-systematic-review-and-meta-analysis/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress