Identification of Adult Liver Transplant Recipients Eligible to Participate in an Immunosuppression Withdrawal Trial Employing Non-Invasive Assessments of Allograft Status
1King's College London, London, United Kingdom, 2Royal Free, London, United Kingdom, 3St-Luc, Brussels, Belgium, 4Addenbrookes, Cambridge, United Kingdom, 5Hospital Clinic, Barcelona, Spain, 6Queen Elizabeth, Birmingham, United Kingdom, 7Royal Infirmary, Edinburgh, United Kingdom, 8Charité, Berlin, Germany, 9Hannover Medical School, Hannover, Germany, 10Leeds NHS Trust, Leeds, United Kingdom, 11UZ Leuven, Leuven, Belgium, 12Hepatology, Newcastle, United Kingdom
Meeting: 2019 American Transplant Congress
Abstract number: D352
Keywords: Immunosuppression, Liver transplantation, Tolerance
Session Information
Session Name: Poster Session D: Tolerance: Clinical Studies
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Liver Immunosuppression Free Trial (LIFT) is a multicentre international prospective randomised controlled trial of biomarker-guided immunosuppression withdrawal in adult liver transplant recipients who meet the following criteria: >3 years post-transplant, normal liver function, no autoimmune or replicative viral disease, no significant inflammatory/fibrotic histological lesions.
*Methods: Between 11/2015 and 11/2018, 151 patients underwent a screening liver biopsy to determine histological eligibility. Additional baseline assessments included liver tissue gene expression, liver stiffness measurement (LSM, FibroScan) and circulating anti-HLA antibodies. Two central pathologists evaluated all biopsies.
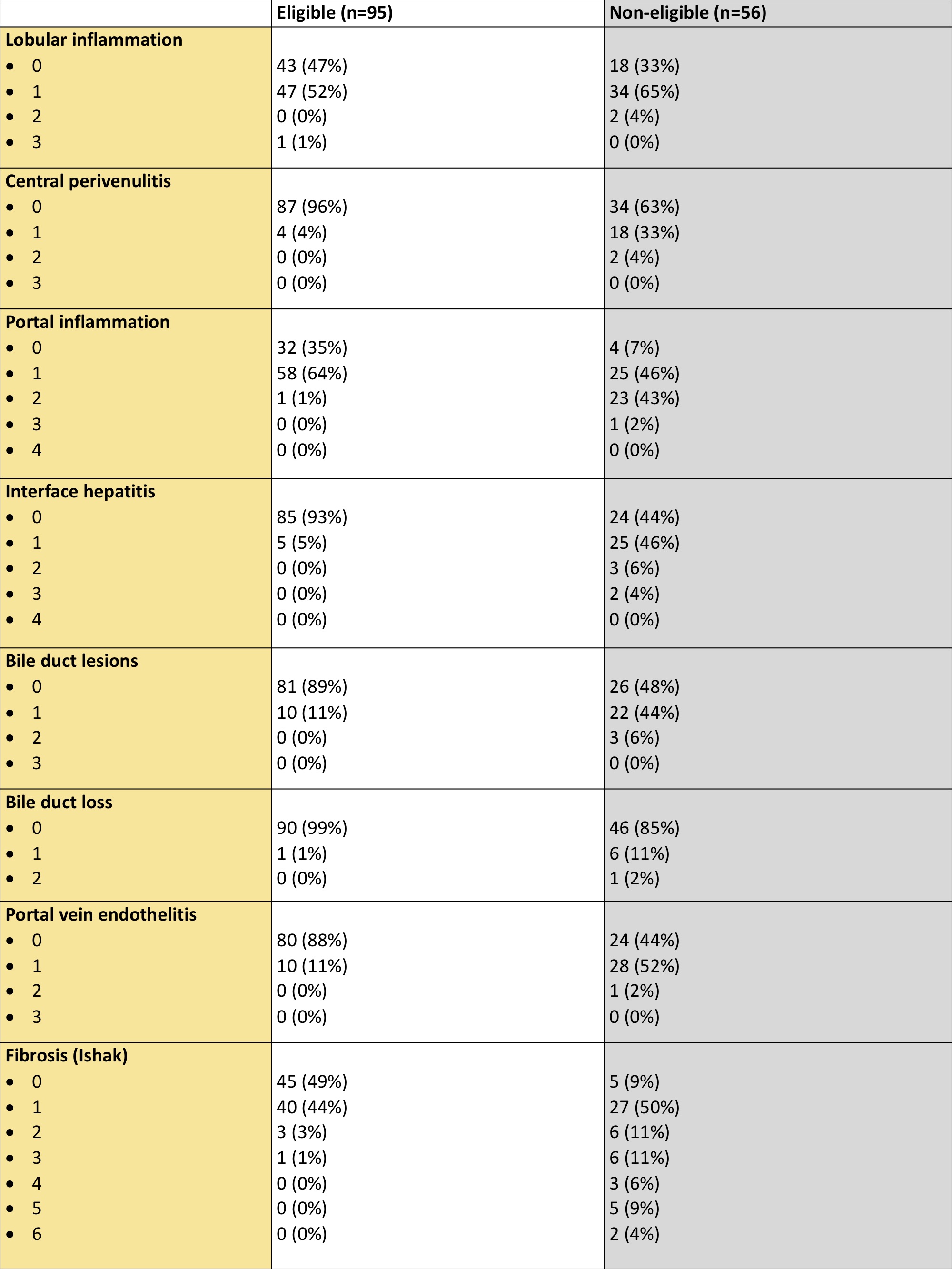
*Results: Out of the 151 biopsies, 95 (63%) met histological eligibility and 56 (37%) did not. Main histological differences between eligible and non-eligible patients are described in Image 1. Non-eligible patients had a higher mean LSM than eligible patients (7,5 vs 4,5 kPa; p=0.001) despite no differences in liver biochemistry. LSM exhibited a good discrimination capacity with AUC=0,84. Each 1 kPa increase in LSM raised the odds ratio of being non-eligible by 2,28 (p=0.001). Moreover, the use of a LSM cut-off of >8,3 kPa identified non-eligibility with PPV=100%, NPV=70%, Sn=31%, and Sp=100%.
*Conclusions: A large proportion of adult stable liver recipients with normal liver tests exhibit subclinical histological abnormalities that preclude their eligibility to participate in an immunosuppression withdrawal trial. In this population, LSM could be a useful non-invasive tool to stratify patients prior to performing surveillance liver biopsies.
To cite this abstract in AMA style:
Vionnet J, Miquel R, Douiri A, Elstad M, Kodela E, Quaglia A, Wall J, Martin PRuiz, Riani EB, Leithead J, Marshall A, Navasa M, Ruiz P, Ferguson J, Simpson K, Pascher A, Jaeckel E, Bishop R, Nevens F, Masson S, Sanchez-Fueyo A. Identification of Adult Liver Transplant Recipients Eligible to Participate in an Immunosuppression Withdrawal Trial Employing Non-Invasive Assessments of Allograft Status [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/identification-of-adult-liver-transplant-recipients-eligible-to-participate-in-an-immunosuppression-withdrawal-trial-employing-non-invasive-assessments-of-allograft-status/. Accessed February 24, 2026.« Back to 2019 American Transplant Congress