Galectin-3 Levels in Pediatric Cholestatic Liver Disease
1Transplant Surgery, University of Colorado, Aurora, CO, 2Pediatric Gastroenterology, Hepatology and Nutrition, Children’s Hospital Colorado, Aurora, CO
Meeting: 2021 American Transplant Congress
Abstract number: 549
Keywords: Infant, Inflammation, Liver, Pediatric
Topic: Basic Science » Biomarker Discovery and Immune Modulation
Session Information
Session Name: Biomarker Discovery and Immune Modulation
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Biliary atresia (BA) and other cholestatic liver diseases (CLD) are the most common indications for liver transplantation in children. Research on galectin-3, a protein involved in immune regulation and fibrosis, in CLD of children is limited. We hypothesize that galectin-3 is significantly elevated in pediatric CLD and in a murine model of BA.
*Methods: A model of BA was induced in neonatal BALB/c mice with intraperitoneal injection of Rhesus rotavirus (RRV) or salt solution (controls) within 24 hours of life. The mice were sacrificed at 2 weeks and serum pooled (3-5 mice per pool). Plasma was collected from pediatric liver transplant recipients with CLD at time of transplant. Plasma from children without liver disease were used for comparison. Galectin-3 levels were measured using ELISA kits. A human galectin-3 level of < 17.8 ng/mL was considered normal, 17.8 - 25.9 ng/mL mildly elevated, and greater than 25.9 ng/mL extremely elevated, as previously defined. Data is presented as mean (standard deviation) and compared using Student’s t-test or Chi-squared test.
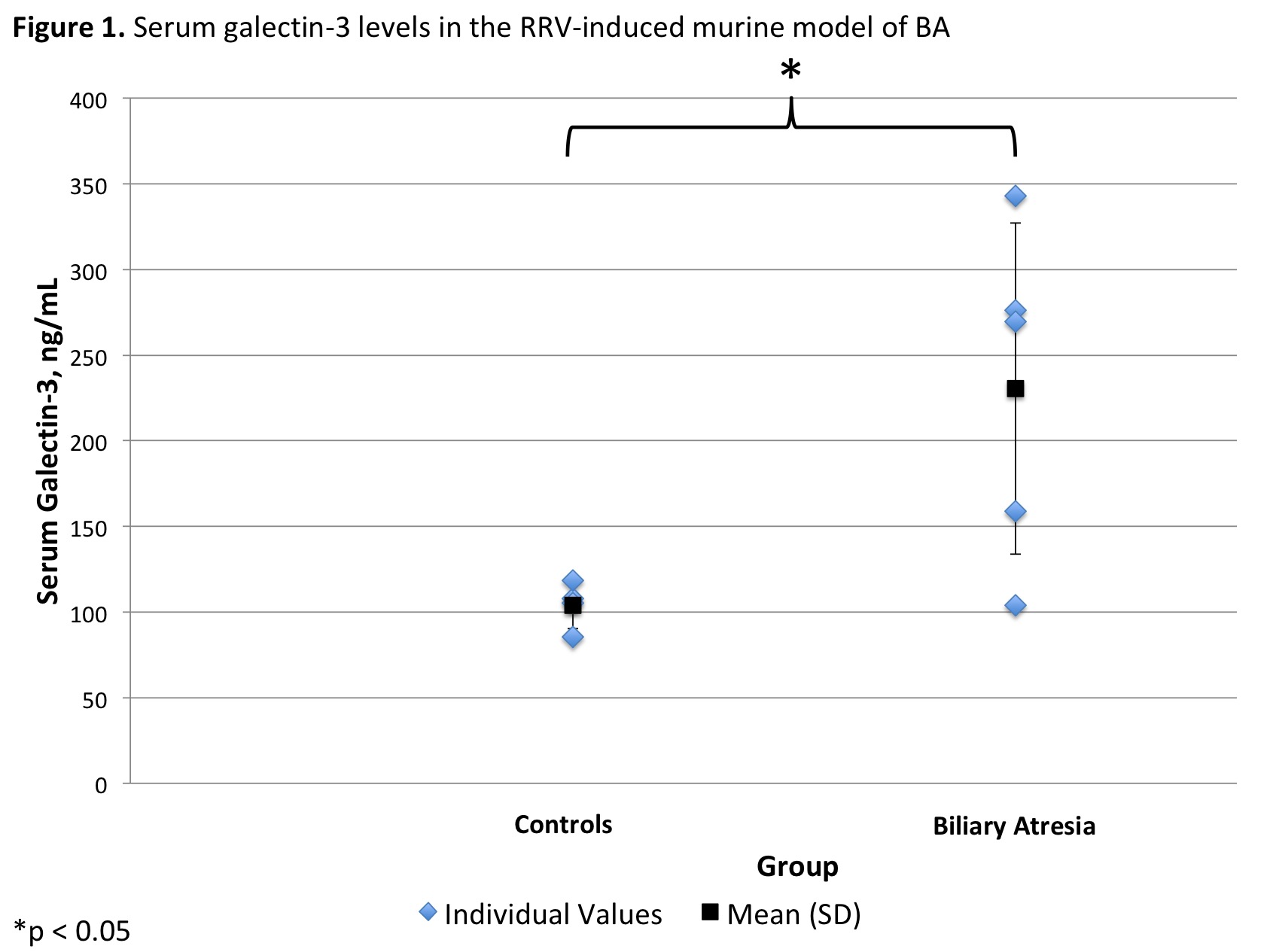
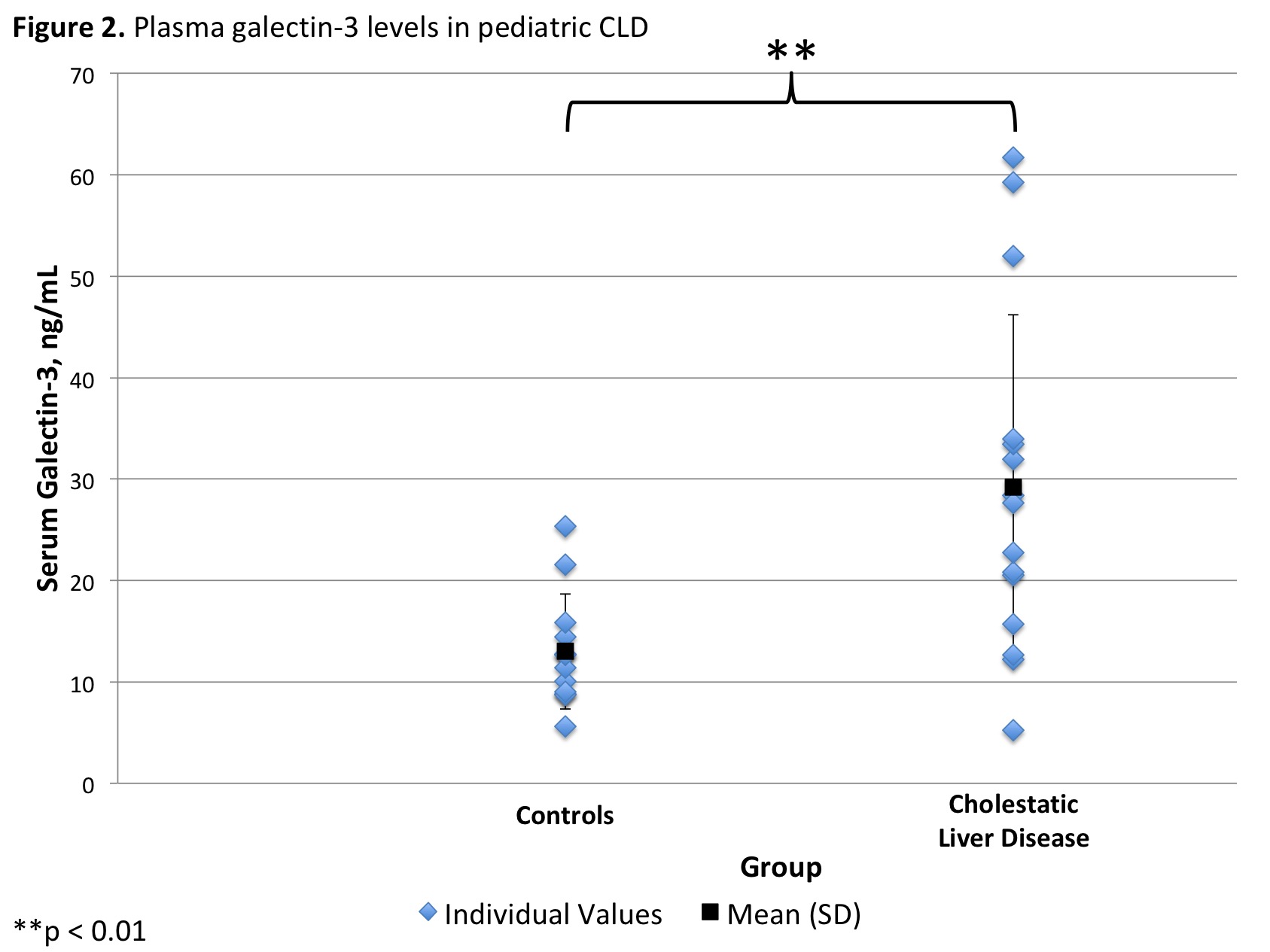
*Results: Serum from 5 pools of BA and 4 pools of control mice was collected. Mean galectin-3 level was 230.4 (±96.8) ng/mL in the BA and 104.1 (±13.8) ng/mL in the control mice groups (p=.04, Figure 1). Plasma was collected from 15 CLD liver transplant recipients (11 BA, 3 Alagille’s Syndrome, 1 PFIC) and 12 controls. The ages of the CLD and control groups were not significantly different (3.8 [±5.4] vs. 4.7 [±5.4] years, respectively; p =0.7). Mean galectin-3 level was 29.2 (±17.0) ng/mL in the CLD and 13.0 (±5.7) ng/mL in the control group (p=.004, Figure 2). In the CLD group, 4 (27%) had normal, 3 (20%) had mildly elevated, and 8 (53%) had extremely elevated galectin-3 levels. This was significantly greater than controls, who had 10 (83%) normal and 2 (17%) mildly elevated galectin-3 levels (p=0.005).
*Conclusions: Galectin-3 is elevated in children with CLD undergoing liver transplant. This elevation is also reflected in a murine model of biliary atresia, which can guide future mechanistic and therapeutic studies of this disease.
To cite this abstract in AMA style:
Yoeli D, Wang Z, Luo Y, Chaidez A, Adams MA, Huang CA, Mack CL, Alvarez NNavarro. Galectin-3 Levels in Pediatric Cholestatic Liver Disease [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/galectin-3-levels-in-pediatric-cholestatic-liver-disease/. Accessed March 9, 2026.« Back to 2021 American Transplant Congress