Galectin-3 and Long-Term Graft Failure in Renal Transplant Recipients: A Prospective Cohort Study
1Division of Nephrology, Department of Internal Medicine, University Medical Center Groningen, Groningen, Netherlands, 2Department of Internal Medicine, University Medical Center Groningen, Groningen, Netherlands, 3Department of Epidemiology, University Medical Center Groningen, Groningen, Netherlands, 4Department of Cardiology, University Medical Center Groningen, Groningen, Netherlands
Meeting: 2019 American Transplant Congress
Abstract number: 410
Keywords: Fibrosis, Graft failure, Renal failure
Session Information
Session Name: Concurrent Session: Kidney Complications: Late Graft Failure III
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Veterans Auditorium
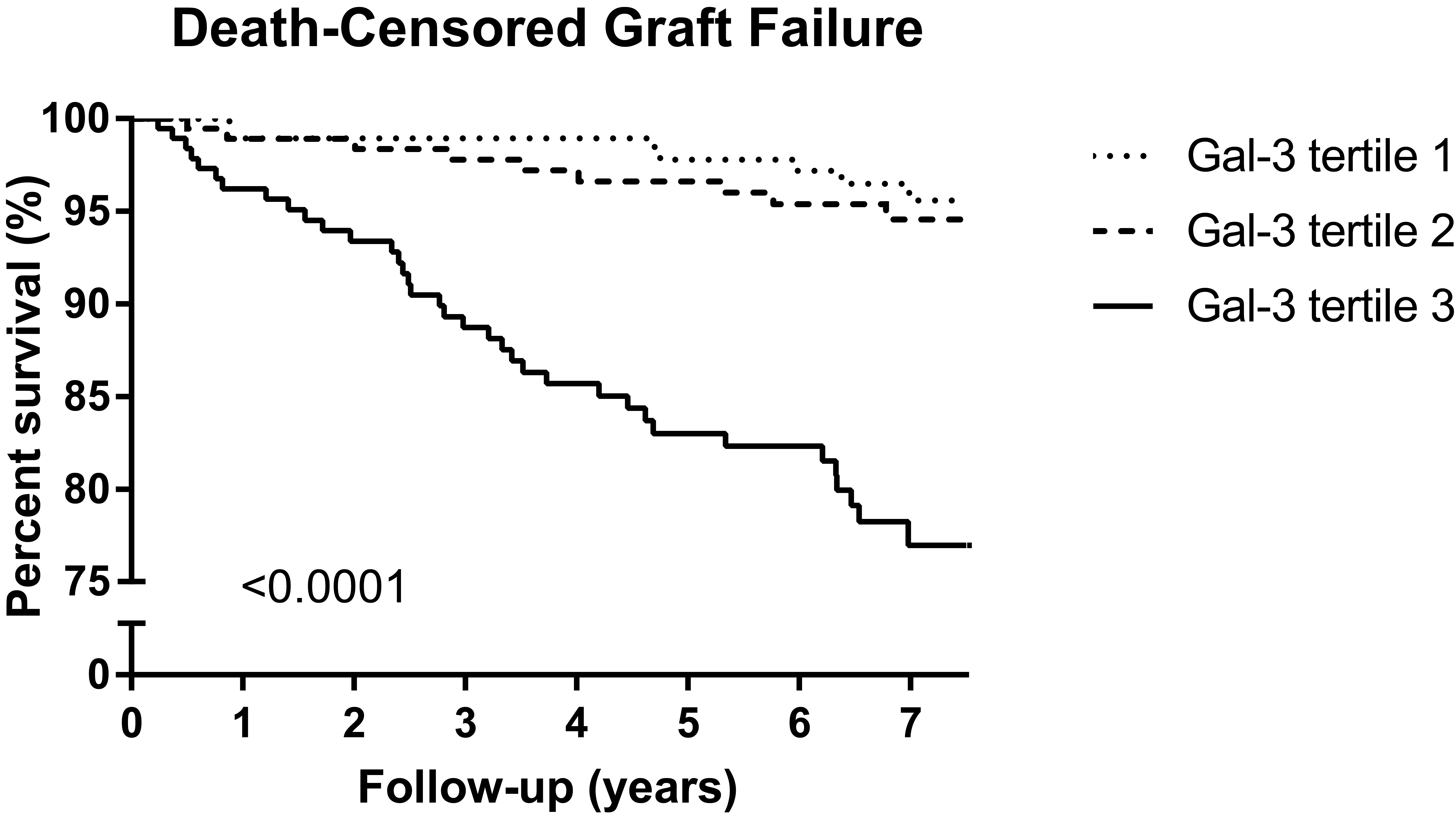
*Purpose: Galectin-3 has been associated with renal fibrosis and decline of renal function. We aimed to determine the association of galectin-3 with long-term risk of death-censored graft failure in a large cohort of extensively phenotyped renal transplant recipients (RTR).
*Methods: We performed a longitudinal cohort study in 561 RTR with a functioning graft ≥1 year, recruited between 2001 and 2003 in a university setting. Galectin-3 levels were determined in serum samples (BG Medicine, Inc, Waltham, MA). Death-censored graft failure was defined as restart of dialysis or re-transplantation. Cox-proportional hazards regression analyses were performed to assess the association of galectin-3 with outcome.
*Results: Baseline median galectin-3 was 21.1 [interquartile range, 17.0‒27.2] ng/mL. During a median follow-up of 6.9 [6.2-7.5] years, 53 RTR developed graft failure. In multivariable-adjusted Cox regression analysis, galectin-3 was associated with death-censored graft failure (hazard ratio, 2.32 per 1-SD increase; 95% confidence interval, 1.68 to 3.20, P<0.001), independent of well-established general and renal transplant-specific risk factors, including estimated Glomerular Filtration Rate and proteinuria.
*Conclusions: In RTR, galectin-3 is elevated and independently associated with long-term risk of death-censored graft failure. Galectin-3 may be helpful to assess prognosis and guide existing therapy. Whether a novel galectin-3-targeted therapy may represent an opportunity to decrease the burden of long-term graft failure in stable RTR requires further studies.
| Model | Gal-3, per 1─SD increase | |
| HR (95%CI) | P | |
| Model 1 | 2.42 (1.90-3.07) | <0.001 |
| Model 2 | 2.32 (1.68-3.20) | <0.001 |
| Model 3 | 2.56 (1.79-3.65) | <0.001 |
| Model 4 | 2.14 (1.51-3.04) | <0.001 |
| Model 1: crude. Model 2: adjustment for recipient age, sex and BMI, donor age and sex, dialysis vintage, type of transplant, time since transplantation time-dependent eGFR and proteinuria. Model 3: model 2 + immunosuppressive therapy. Model 4: model 2 + cardiovascular risk factors. | ||
To cite this abstract in AMA style:
Sotomayor CG, Keyzer CA, Anderson JL, Gans RO, Nolte IM, Borst MHde, Berger SP, Navis GJ, Boer RAde, Bakker SJ. Galectin-3 and Long-Term Graft Failure in Renal Transplant Recipients: A Prospective Cohort Study [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/galectin-3-and-long-term-graft-failure-in-renal-transplant-recipients-a-prospective-cohort-study/. Accessed February 16, 2026.« Back to 2019 American Transplant Congress