Follow Up of Patients Treated with the IgG Endopeptidase (IdeS) for Desensitization and HLA Incompatible (HLAi) Kidney Transplantation
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2Pathology & Laboratory Medicine, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2018 American Transplant Congress
Abstract number: 523
Keywords: Alloantibodies, Highly-sensitized, HLA antibodies, IgG
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: General Considerations - 2
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Room 6A
Introduction:Donor-specific antibodies (DSAs) create an immunologic barrier to transplantation. This prevents transplantation for a significant percentage of highly-HLA sensitized patients (HS). Current desensitization therapies are limited and may be ineffective in the most HS patients. The IgG degrading enzyme derived from Streptococcus pyogenes (IdeS) cleaves human IgG into F(ab')2 and Fc fragments resulting in inhibition of CDC and ADCC, possibly limiting DSA pathogenicity. Recently,(N Engl J Med.2017;377(5):442-453) we reported on outcomes of two phase I/II clinical trials of IdeS. Here, we report on longer-term follow up of patients in the U.S. cohort. Patients & Methods: Eligible patients were HS 18 to 70 years awaiting kidney transplantation. All patients exhibited extensive sensitization with a median cPRA of 95%. Acceptance criteria for HLA-incompatible organs included a negative CDC crossmatch, a negative flow cytometry crossmatch or a positive T- and B- cell flow crossmatch ~250 channel shifts or less and usually DSA+. Patients meeting the criteria outlined above were entered in the U.S. study (NCT02426684). Results: A total of 17 patients were transplanted from June-2015 -July 2017. Pre- & post-transplant desensitization included IVIg + anti-CD20. Data summarizing outcomes including Banff biopsy scores, DSA levels and outcomes are shown below: 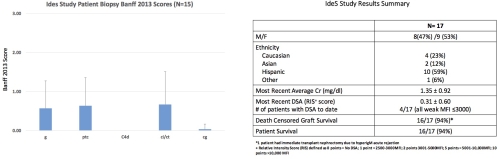 Graft and patient survival at a mean 18.76±5.6 M post-IdeS transplant were 94%. Rebound DSA responses were rare and of low MFI values with only 4 patients demonstrating DSAs, all with MFIs ≤3000. Biopsies were performed in 15 patients. All but 3 showed no or findings "suspicious" for ABMR that did not meet Banff criteria. No patient treated with IdeS had C4d+. Conclusions: Patients desensitized with IdeS and transplanted with HLAi kidneys show good renal function and minimal evidence of allo-specific responses at mean 18.56 M post-transplant.
Graft and patient survival at a mean 18.76±5.6 M post-IdeS transplant were 94%. Rebound DSA responses were rare and of low MFI values with only 4 patients demonstrating DSAs, all with MFIs ≤3000. Biopsies were performed in 15 patients. All but 3 showed no or findings "suspicious" for ABMR that did not meet Banff criteria. No patient treated with IdeS had C4d+. Conclusions: Patients desensitized with IdeS and transplanted with HLAi kidneys show good renal function and minimal evidence of allo-specific responses at mean 18.56 M post-transplant.
CITATION INFORMATION: Jordan S., Choi J., Huang E., Najjar R., Peng A., Zhang X., Louie S., Haas M., Sethi S., Kim I., Vo A. Follow Up of Patients Treated with the IgG Endopeptidase (IdeS) for Desensitization and HLA Incompatible (HLAi) Kidney Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Jordan S, Choi J, Huang E, Najjar R, Peng A, Zhang X, Louie S, Haas M, Sethi S, Kim I, Vo A. Follow Up of Patients Treated with the IgG Endopeptidase (IdeS) for Desensitization and HLA Incompatible (HLAi) Kidney Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/follow-up-of-patients-treated-with-the-igg-endopeptidase-ides-for-desensitization-and-hla-incompatible-hlai-kidney-transplantation/. Accessed February 28, 2026.« Back to 2018 American Transplant Congress
