Five-Year Follow-Up from a Prospective, Randomized Trial of Belatacept-Based, CNI Free/Early Steroid Withdrawal Regimens
1University of Cincinnati, Cincinnati, OH, 2Christ Hospital, Cincinnati, OH
Meeting: 2022 American Transplant Congress
Abstract number: 410
Keywords: Co-stimulation, Graft survival, Immunosuppression, Kidney transplantation
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression
Session Type: Rapid Fire Oral Abstract
Date: Tuesday, June 7, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:40pm-3:50pm
Presentation Time: 3:40pm-3:50pm
Location: Hynes Room 302
*Purpose: A prospective, randomized, multi-center trial designed to compare two belatacept (BELA)-based, calcineurin inhibitor-free (CNI), early corticosteroid withdrawal (ESW) regimens with a tacrolimus (TAC)-based ESW regimen. Long-term safety and efficacy were analyzed at five years for the 2 highest enrolling centers.
*Methods: This study was conducted under an FDA IND at eight sites. 5 year results from 168 patients at the two lead enrolling centers were analyzed. Adult kidney transplant (KTx) recipients of living and deceased donor allografts all received mycophenolate therapy and a five-day steroid taper. Patients were randomized to three treatment groups: alemtuzumab + BELA (Group A), rATG + BELA (Group B), and rATG + TAC (Group C). Primary endpoint was a composite of patient death, graft loss, or eGFR < 45ml/min/1.73m2. Randomized and transplanted patients constituted the intent to treat (ITT) population. Per protocol analyses were conducted on patients who did not experience major protocol deviations in immunosuppression regimens. Imputations for graft loss or death were made by imputing 0 for eGFR and by carrying forward last recorded eGFR value for patients lost to follow up.
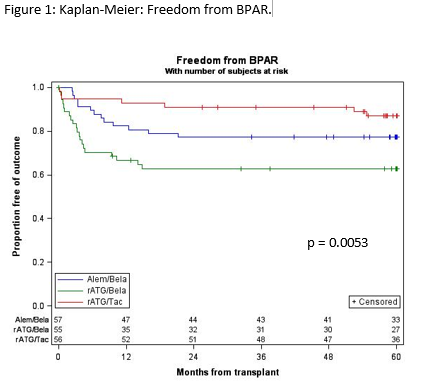
*Results: Enrollment was completed on 12/15/16. Demographics were similar across all groups with majority being living donors, primary transplants, non-African American race, and low DGF rates. ITT analyses (Table 1) and per protocol analyses (Table 2) are presented. Of note, no additional patients with rejection were observed in 112 patients on BELA beyond 2 years; whereas, 3/56 (5.4%) patients on TAC had rejection after 2 years (p=0.12).
*Conclusions: Long-term follow-up of this trial indicates that belatacept-based regimens with ESW provide: 1) patient and graft survival rates that are comparable to the control tacrolimus-based regimen, 2) a trend towards improved kidney function out to 5 years, and 3) despite higher early BPAR rates, BPAR beyond 2 years was not observed in belatacept-treated patients after 2 years, whereas 5.4% of tacrolimus-treated patients experienced rejection beyond 2 years.
To cite this abstract in AMA style:
Hames H, Shields AR, Yanqui E, Christianson A, Kremer J, Govil A, Woodle ES, Alloway RR. Five-Year Follow-Up from a Prospective, Randomized Trial of Belatacept-Based, CNI Free/Early Steroid Withdrawal Regimens [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/five-year-follow-up-from-a-prospective-randomized-trial-of-belatacept-based-cni-free-early-steroid-withdrawal-regimens/. Accessed July 18, 2025.« Back to 2022 American Transplant Congress