First Report of Human Liver Transplantation After Normothermic Machine Perfusion (NMP) in the United States.
Cleveland Clinic, Cleveland
Meeting: 2017 American Transplant Congress
Abstract number: 276
Keywords: Liver preservation, Liver transplantation, Machine preservation, Safety
Session Information
Session Name: Concurrent Session: Organ Perfusion Strategies
Session Type: Concurrent Session
Date: Monday, May 1, 2017
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:42pm-2:54pm
Presentation Time: 2:42pm-2:54pm
Location: E353C
NMP is a novel preservation method for liver grafts. We transplanted 10 human livers after NMP to test the safety and feasibility of NMP on a device developed in our institution. Livers included 8 from donors after brain death (DBD) and 2 after circulatory death (DCD). Cold ischemia time before NMP was 1hrs32mins to 3hrs57mins. NMP time was 4hrs to 7hrs52mins. Livers were perfused through portal vein and hepatic artery in physiologic flow rates and pressures with perfusate based on matched human packed red blood cells and fresh frozen plasma. During NMP, bile production was 3-13 ml/hr of DBD livers and 1ml/hr of DCD livers. All livers had lactate clearance. 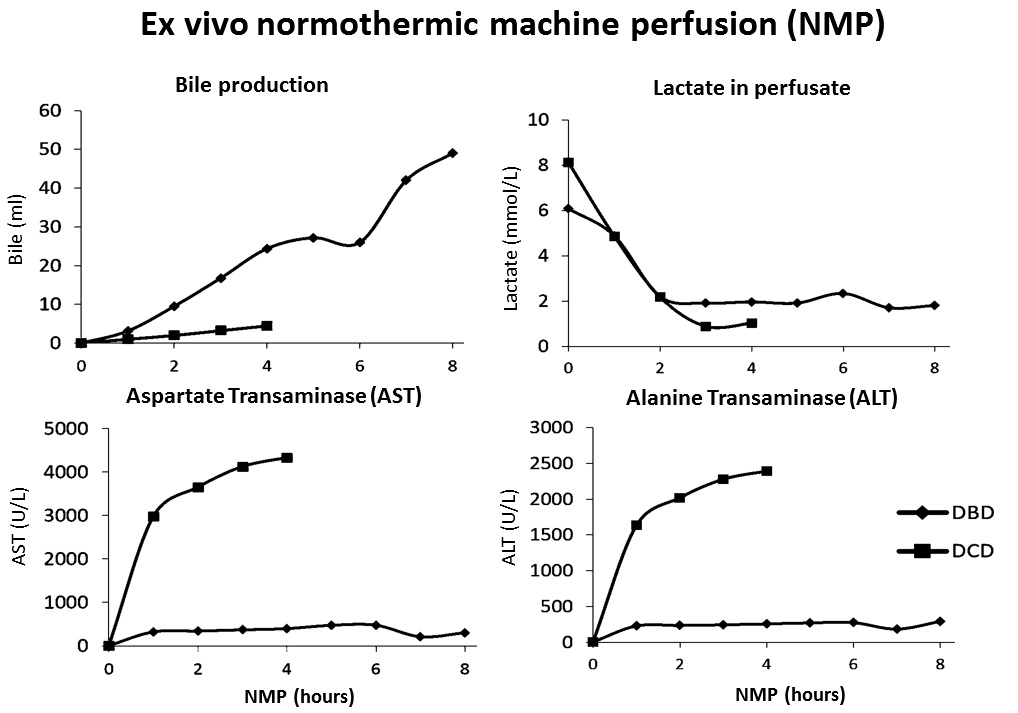 The alanine and aspartate aminotransferase (ALT, AST) in perfusate at the end of NMP had correlation (both p=0.001) to their peak values in the first 7 post-operative days (POD).
The alanine and aspartate aminotransferase (ALT, AST) in perfusate at the end of NMP had correlation (both p=0.001) to their peak values in the first 7 post-operative days (POD).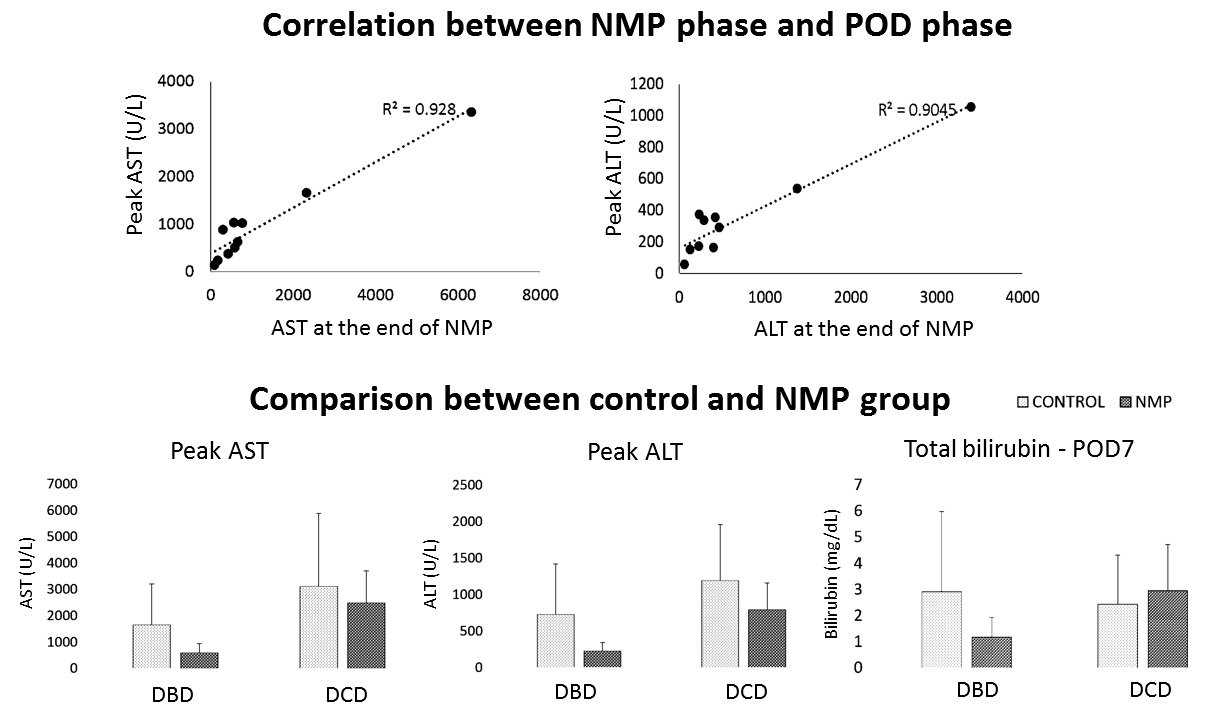 NMP livers were compared to historic liver transplant controls preserved by cold storage matched (1:4) by donor and recipient age, donor risk index, MELD score, total preservation time. Early allograft dysfunction (EAD) was defined as total bilirubin>10mg/dL or International Normal Ratio (INR)>1.6 at POD7, or peak ALT or AST>2000 U/L until POD7. EAD ratio was 10% in NMP group (a DCD liver) but was 36.8% of the controls (p=0.13). NMP group had significant difference to the controls on peak AST (p=0.001) and ALT (p=0.001) and total bilirubin at POD7 (p=0.007) of DBD livers, but not DCD livers (all p>0.05). These results indicated the safety and feasibility as well as potential benefits of NMP technique on protecting and predicting liver function.
NMP livers were compared to historic liver transplant controls preserved by cold storage matched (1:4) by donor and recipient age, donor risk index, MELD score, total preservation time. Early allograft dysfunction (EAD) was defined as total bilirubin>10mg/dL or International Normal Ratio (INR)>1.6 at POD7, or peak ALT or AST>2000 U/L until POD7. EAD ratio was 10% in NMP group (a DCD liver) but was 36.8% of the controls (p=0.13). NMP group had significant difference to the controls on peak AST (p=0.001) and ALT (p=0.001) and total bilirubin at POD7 (p=0.007) of DBD livers, but not DCD livers (all p>0.05). These results indicated the safety and feasibility as well as potential benefits of NMP technique on protecting and predicting liver function.
CITATION INFORMATION: Liu Q, Soliman B, Iuppa G, Nassar A, Hassan A, Diago Uso T, Hashimoto K, Aucejo F, Fujiki M, Eghtesad B, Cywinski J, Irefin S, Fung J, Abu-Elmagd K, Miller C, Quintini C. First Report of Human Liver Transplantation After Normothermic Machine Perfusion (NMP) in the United States. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Liu Q, Soliman B, Iuppa G, Nassar A, Hassan A, Uso TDiago, Hashimoto K, Aucejo F, Fujiki M, Eghtesad B, Cywinski J, Irefin S, Fung J, Abu-Elmagd K, Miller C, Quintini C. First Report of Human Liver Transplantation After Normothermic Machine Perfusion (NMP) in the United States. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/first-report-of-human-liver-transplantation-after-normothermic-machine-perfusion-nmp-in-the-united-states/. Accessed March 5, 2026.« Back to 2017 American Transplant Congress
