Ex Vivo Normothermic Perfusion Of Discarded Human Kidneys Reveals Cold-storage-induced Renal Fibrinogen And Provides A Platform For Therapeutic Repair
1Surgery, Yale University & University of Cambridge, New Haven, CT, 2Surgery, University of Cambridge, Cambridge, United Kingdom, 3Biomedical Engineering, Yale University, New Haven, CT, 4Surgery - Transplantation, Yale University, New Haven, CT
Meeting: 2019 American Transplant Congress
Abstract number: 603
Keywords: Donors, marginal, Image analysis, Kidney transplantation, Renal ischemia
Session Information
Session Name: Concurrent Session: Ischemia Reperfusion & Organ Rehabilition III
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: Room 313
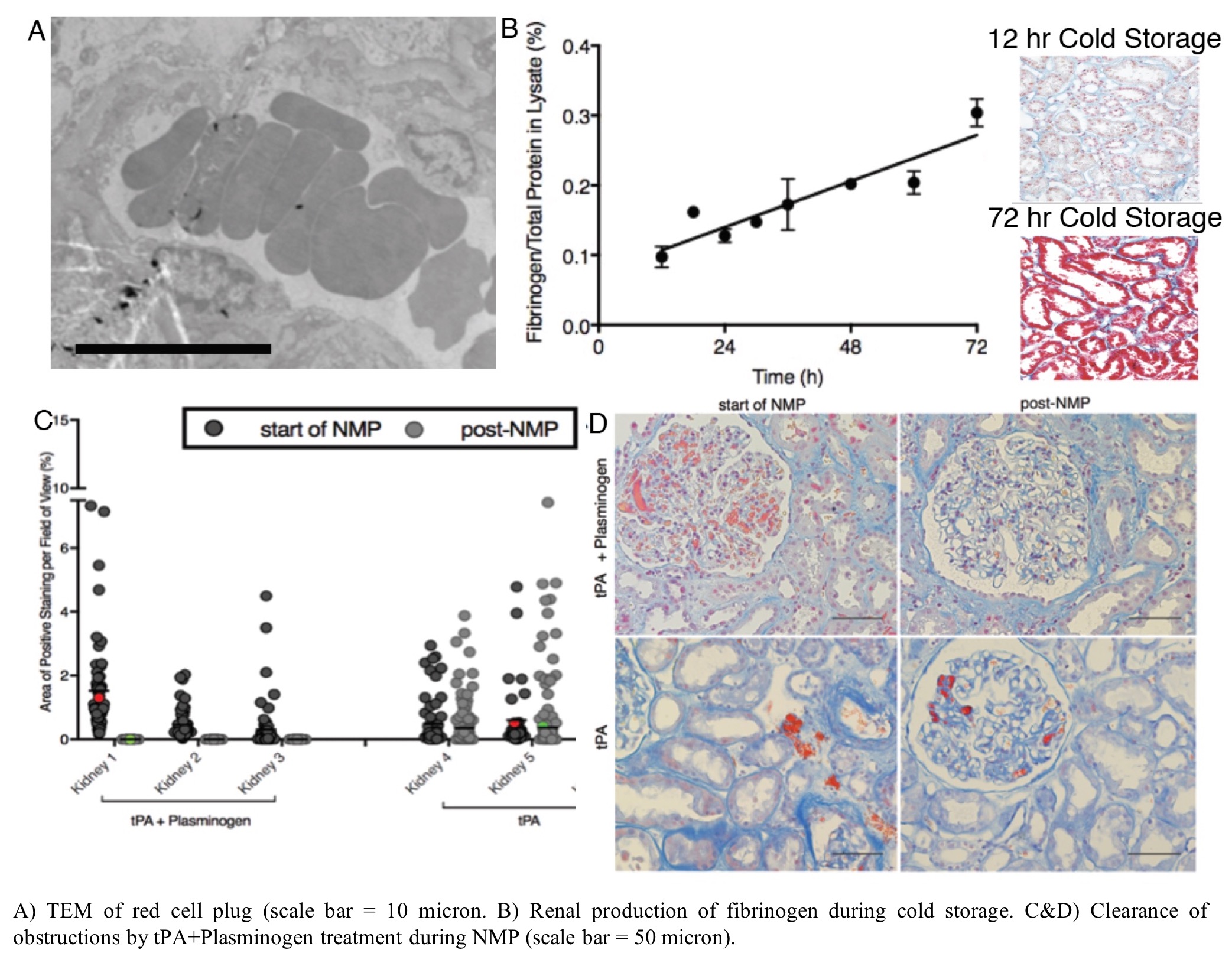
*Purpose: The purpose of this study was to: 1) determine the cause of microvascular obstructions that arise during ex vivo normothermic perfusion (NMP) of human kidney; and 2) develop a therapeutic strategy to clear these obstructions to restore perfusion in marginal human kidney allografts.
*Methods: A series of 18 discarded donor human kidneys were employed. 3 organs were used to assess renal production of fibrinogen during cold storage. 15 additional organs underwent NMP following established protocols. Organs were biopsied and assessed for renal fibrinogen with histological and immunofluoresence staining. Treatment with tissue plasminogen activator (tPA) and plasminogen was used to resolve microvascular obstructions during NMP. Biopsies collected before and after treatment were sectioned and stained for microvascular obstructions for analysis by quantitative microscopy. TEM was used to confirm the composition of the microvascular obstructions. Perfusate samples were tested to confirm fibrinolysis and physiological assessment of ex vivo kidney function was performed.
*Results: In the absence of traditional clotting factors (using serum-free washed red blood cells), microvascular obstructions formed during NMP and were found to be red cell plugs rich in fibrinogen with a rouleaux like presentation. Fibrinogen was not present in the washed red blood cells prior to NMP, but rapidly appeared in the perfusate following 15 min of normothermic perfusion. Histological and immunofluorescent staining combined with protein elisa confirmed that tubular epithelial cells produce fibrinogen locally in response to cold storage. NMP elicits release of the epithelial fibrinogen into renal vasculature leading to the red cell plugs. Treatment with tissue plasminogen activator (tPA) in combination with plasminogen efficiently cleared these obstructions in all kidneys and restored perfusion in even highly obstructed organs. This treatment reduced markers of inflammation (e.g. IL6) and improved physiological parameters (e.g. urine production).
*Conclusions: These results reveal a new mechanism of local renal production of fibrinogen in response to cold storage. Normothermic perfusion conditions induces release of fibrinogen into the vasculature, which can cause microvascular obstructions by cross linking red cells. NMP allows this process to happen in a controlled ex vivo environment where therapeutics (i.e. tPA+plasminogen) can be used to clear these obstructions and restore perfusion. This approach also avoids release of the fibrinogen in the organ recipient, where a thrombotic response could elicit delayed graft function and myriad other complications. Collectively our results demonstrate the power of NMP as: 1) a preclinical research tool to investigate the pathophysiology of ischemic injury; and 2) a platform for therapeutic repair of marginal human organs.
To cite this abstract in AMA style:
DiRito J, Hosgood SA, Reschke M, Nicholson ML, Tietjen GT. Ex Vivo Normothermic Perfusion Of Discarded Human Kidneys Reveals Cold-storage-induced Renal Fibrinogen And Provides A Platform For Therapeutic Repair [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/ex-vivo-normothermic-perfusion-of-discarded-human-kidneys-reveals-cold-storage-induced-renal-fibrinogen-and-provides-a-platform-for-therapeutic-repair/. Accessed March 3, 2026.« Back to 2019 American Transplant Congress